Abstract
In order to study the characteristics of norovirus in Gwangju metropolitan city, We examined norovirus in 13,931 fecal specimens collected through five years (2008–2012) from children admitted with a chief complain of acute diarrhea. Among a total of norovirus (NoV) was most frequently detected (3,025 cases, 21.7%). Concerning the frequency of virus detected by month, NoV tended to break out frequently from October to March in the following year. NoV was detected most highly in 0~3 year infants. Through examinations on NoV genotypes, among 3,025 cases that turned out to be positive, the genotypes of 2,652 cases were determined with various results including 13 types of GI and 17 types of GII. The results of analysis on GI genotypes were as follows: GI-4 (21.9%), GI-2 (15.2%), and GI-6 (10.5%). GII genotypes were as follows: GII-4 (63.9%), GII-3 (18.9%), GII-8 (4.2%), GII-2 (3.9%), GII-6 (3.3%), and GII-1 (1.9%). Eight types of variants for GII-4 genotype (427 cases) were identified. The majority of the GII-4 variants was GII-4_Farmington (181 cases, 42.4%), which peaked in 2012, while GII-4_2008b (173 cases, 40.5%) showed a high prevalence in 2011. Concerning the circulation of variants, as many as eight types of GII-4 variants were identified in 2012, showing more varieties than in other years. Therefore, this study can be used as fundamental data for the development of vaccine candidate for the prevention of viral diarrheal diseases with high-incidence.
Go to : 
REFERENCES
1). Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010; 375:1969–87.

2). Wardlaw T, Salama P, Brocklehurst C, Chopra M, Mason E. Diarrhoea: why children are still dying and what can be done. Lancet. 2010; 375:870–2.

3). Green KY, Ando T, Balayan MS, Berke T, Clarke IN, Estes MK, et al. Taxonomy of the Caliciviruses. J Infect Dis. 2000; 181:322–30.

4). Koopmans M. Food-borne norovirus outbreaks: a nuisance or more than that? Wien Klin Wochenschr. 2005; 117:789–91.

5). Vinje J, Hamidjaja RA, Sobsey MD. Development and application of a capsid VP1 (region D) based reverse transcription PCR assay for genotyping of genogroup I and II noroviruses. J Virol Methods. 2004; 116:109–17.
6). Green KY. Caliciviridae: the noroviruses. Knipe DM, Howley PM, editors. Fields Virology. Philadelphia: Lippincott Williams & Wilkins;2007. p. 949–78.
7). Siebenga JJ, Vennema H, Zheng DP, Vinjé J, Lee BE, Pang XL, et al. Norovirus illness is a global problem: emergence and spread of Norovirus GII.4 Variants, 2001–2007. J Infect Dis. 2009; 200:802–12.

8). Donaldson EF, Lindesmith LC, Lobue AD, Baric RS. Viral shape-shifting: norovirus evasion of the human immune system. Nat Rev Microbiol. 2010; 8:231–41.

9). Zheng DP, Widdowson MA, Glass RI, Vinjé J. Molecular epidemiology of genogroup II-genotype 4 noroviruses in the United States between 1994 and 2006. J Clin Microbiol. 2010; 48:168–77.

11). Marshall JA, Hellard ME, Sinclair MI, Fairley CK, Cox BJ, Catton MG, et al. Incidence and characteristics of endemic Norwalk-like virus-associated gastroenteritis. J Med Virol. 2003; 69:568–78.

12). Blanton LH, Adams SM, Beard RS, Wei G, Bulens SN, Widdowson MA, et al. Molecular and epidemiologic trends of caliciviruses associated with outbreaks of acute gastroenteritis in the United States, 2000–2004. J Infect Dis. 2006; 193:413–21.

13). Svraka S, Duizer E, Vennema H, de Bruin E, van der Veer B, Dorresteijn B, et al. Etiological role of viruses in outbreaks of acute gastroenteritis in the Netherlands from 1994 through 2005. J Clin Microbiol. 2007; 45:1389–94.

14). Bruggink L, Sameer R, Marshall J. Molecular and epidemiological characteristics of norovirus associated with community-based sporadic gastroenteritis incidents and norovirus outbreaks in Victoria, Australia, 2002–2007. Intervirology. 2010; 53:167–72.

15). Gonzalez-Galan V, Sánchez-Fauqier A, Obando I, Montero V, Fernandez M, Torres MJ, et al. High prevalence of community-acquired norovirus gastroenteritis among hospitalized children: a prospective study. Clin Microbiol Infect. 2011; 17:1895–9.

16). Lopman BA, Hall AJ, Curns AT, Parashar UD. Increasing rates of gastroenteritis hospital discharges in US adults and the contribution of norovirus, 1996–2007. Clin Infect Dis. 2011; 52:466–74.

17). Wiegering V, Kaiser J, Tappe D, Weissbrich B, Morbach H, Girschick HJ. Gastroenteritis in childhood: a retrospective study of 650 hospitalized pediatric patients. Int J Infect Dis. 2011; 15:401–7.

18). De Leon R, Matsui SM, Baric RS, Herrmann JE, Blacklow NR, Greenberg HB, et al. Detection of Norwalk virus in stool specimens by reverse transcriptase-polymerase chain reaction and nonradioactive oligoprobes. Clin Microbiol. 1992; 30:3151–7.

19). Burton-MacLeod JA, Kane EM, Beard RS, Hadley LA, Glass RI, Ando T. Evaluation and comparison of two commercial enzyme linked immunosorbent assay kits for detection of antigenically diverse human noroviruses in stool samples. J Clin Microbiol. 2004; 42:2587–95.
20). Schwab KJ, Neill FH, Le Guyader F, Estes MK. Atmar RL. Development of a reverse transcription-PCR-DNA enzyme immunoassay for detection of Norwalk-like viruses and hepatitis A virus in stool and shellfish. Appl Environ Microbiol. 2001; 67:742–9.
21). Yoda T, Suzuki Y, Terano Y, Yamazaki K, Sakon N, Kuzuguchi T, et al. Precise characterization of norovirus (Norwalk-like virus)-specific monoclonal antibodies with broad reactivity. J Clin Microbiol. 2003; 41:2367–71.

22). Nilsson M, Hedlund KO, Thorhagen M, Larson G, Johansen K, Ekspong A, et al. Evolution of human calicivirus RNA in vivo: accumulation of mutations in the protruding P2 domain of the capsid leads to structural changes and possibly a new phenotype. J Virol. 2003; 77:13117–24.
23). Gallimore CI, Green J, Lewis D, Richards AF, Lopman BA, Hale AD, et al. Diversity of noroviruses cocirculating in the north of England from 1998 to 2001. J Clin Microbiol. 2004; 42:1396–401.

24). Dingle KE. Mutation in a Lordsdale norovirus epidemic strain as a potential indicator of transmission routes. J Clin Microbiol. 2004; 42:3950–7.

25). Siebenga JJ, Vennema H, Zheng DP, Vinjé J, Lee BE, Pang XL, et al. Norovirus illness is a global problem: emergence and spread of norovirus GII.4 variants, 2001–2007. J Infect Dis. 2009; 200:802–12.

26). Zheng DP, Widdowson MA, Glass RI, Vinjé J. Molecular epidemiology of genogroup II-genotype 4 noroviruses in the United States between 1994 and 2006. J Clin Microbiol. 2010; 48:168–77.

27). Ham HJ, Oh SA, Kim CK, Jang JI, Jo SJ, Choi SM. Molecular Characteristics of Human Noroviruses Genogroup I and Genogroup II Detected in Acute Gastroenteritis Patients in Seoul. J Environ Health Sci. 2012; 38:57–65.

28). Cho HG, Lee SG, Kim JE, Yu KS, Lee DY, Park PH, et al. Molecular Epidemiology of norovirus GII.4 variants in children under 5 years with sporadic acute gastroenteritis in South Korea during 2006–2013. J Clin Virol. 2014; 61:340–4.

29). Kim YJ, Lee MG, Kam SK. Characteristics of norovirus occurrence in Jeju. J of Environ Sci Inter. 2014; 23:219–29.

30). Curtis V, Cairncross S. Effect of washing hands with soap on diarrhoea risk in the community: A systematic review. Lancet Infect Dis. 2003; 3:275–81.

31). Lee JI, Park SH, Kim MS, Oh YH, Yu IS, Choi BH, et al. Surveillance of acute gastroenteritis in Seoul, Korea, During May 2004 and June 2007. J Bacteriol Virol. 2009; 39:363–71.

32). Vantarakis A, Mellou K, Spala G, Kokkinos P, Alamanos Y. A gastroenteritis outbreak caused by noroviruses in Greece. Int J Environ Res Public Health. 2011; 8:3468–78.

33). Okada M, Ogawa T, Kaiho I, Shinozaki K. Genetic analysis of noroviruses in Chiba Prefecture, Japan, between 1999 and 2004. J Clin Microbiol. 2005; 43:4391–401.

34). Fang ZY, Xie HP, Lv HX, Zhang Q, Duan ZJ, Steele D, et al. Investigation of human calicivirus (HuCV) diarrhea among infantile and young children in China, 1999~2005. Bing Du Xue Bao. 2007; 23:9–15.
35). Blanton LH, Adams SM, Beard RS, Wei G, Bulens SN, Widdowson MA, et al. Molecular and epidemiologic trends of caliciviruses associated with outbreaks of acute gastroenteritis in the United States, 2000–2004. J Infet Dis. 2006; 193:413–21.

36). Ren Z, kong Y, Wang J, Wang Q, Huang A, Xu H. Etiological study of enteric viruses and the genetic diversity of norovirus, sapovirus, adenovirus and astrovirus in children with diarrhea in Chongqing, China. BMC Infect Dis. 2013; 13:412.

37). Kim NH, Park EH, Park YK, Min SK, Jin SH, Park SH. Study on norovirus genotypes in Busan, Korea. J life Sci. 2011; 21:845–50.

38). Gong YW, Oh BY, Kim HY, Lee MY, Kim YH, Go JM, et al. Molecular epidemiologic investigation of norovirus infections in Incheon city, Korea, from 2005 to 2007. J Bacteriol Virol. 2008; 38:249–57.

39). Kim EJ, Park SH, Song MO, Kim MS, Kim MS, Kim MY, et al. Genetic distribution of human noroviruses detected from acute gastroenteritis patients in Seoul. Kor J Microbiol. 2008; 44:135–9.
40). Hansman GS, Doan LT, Kguyen TA, Okitsu S, Katayama K, Ogawa S, et al. Detection of norovirus and sapovirus infection among children with gastroenteritis in Ho Chi Minh City, Vietnam. Arch Virol. 2004; 149:1673–88.

41). Hansman GS, Katayama K, Maneekarn N, Peerakome S, Khamrin P, Tonusin S, et al. Genetic diversity of norovirus and sapovirus in hospitalized infants with sporadic cases of acute gastroenteritis in Chiang Mai, Thailand. J Clin Microbiol. 2004; 42:1305–7.

42). Wu FT, Oka T, Katayama K, Wu HS, Donald Jiang DS, Miyamura T, et al. Genetic diversity of noroviruses in Taiwan between November 2004 March 2005. Arch Virol. 2006; 151:1319–27.
43). Dey SK, Nguyen TA, Phan TG, Nishio O, Salim AF, Rahman M, et al. Molecular and epidemiological trend of norovirus associated gastroenteritis in Dhaka Ciry, Bangladesh. J Clin Virol. 2007; 40:218–23.
44). Sai L, Sun J, Shao L, Chen S, Liu H, Ma L. Epidemiology and clinical features of rotavirus and norovirus infection among children in Ji'nan, China. Virol J. 2013; 10:302.

45). Patel MM, Hall AJ, Vinjé J, Parashar UD. Noroviruses: a comprehensive review. J Clin Virol. 2009; 44:1–8.

46). van Beek J, Ambert-balay K, Botteldoorn N, Eden JS, Fonager J, Hewitt J, et al. Indications for worldwide increased norovius activity associated with emergence of a new variant of genotype II.4, late 2012. Euro Surveill. 2013; 18:8–9.

47). Tra My PV, Lam HM, Thompson CN, Phuc HL, Tuyet PT, Vinh H, et al. The dynamics of GII. 4 Norovirus in Ho Chi Mihn City, Vietnam. Infect Genet Evol. 2013; 18:335–43.
48). Widdowson MA, Cramer EH, Hadley L, Bresee JS, Beard RS, Bulens SN, et al. Outbreaks of acute gastroenteritis on cruise ships and on land: identification of a predominant circulating strain of norovirus United States, 2002. J Infect Dis. 2004; 190:27–36.
Go to : 
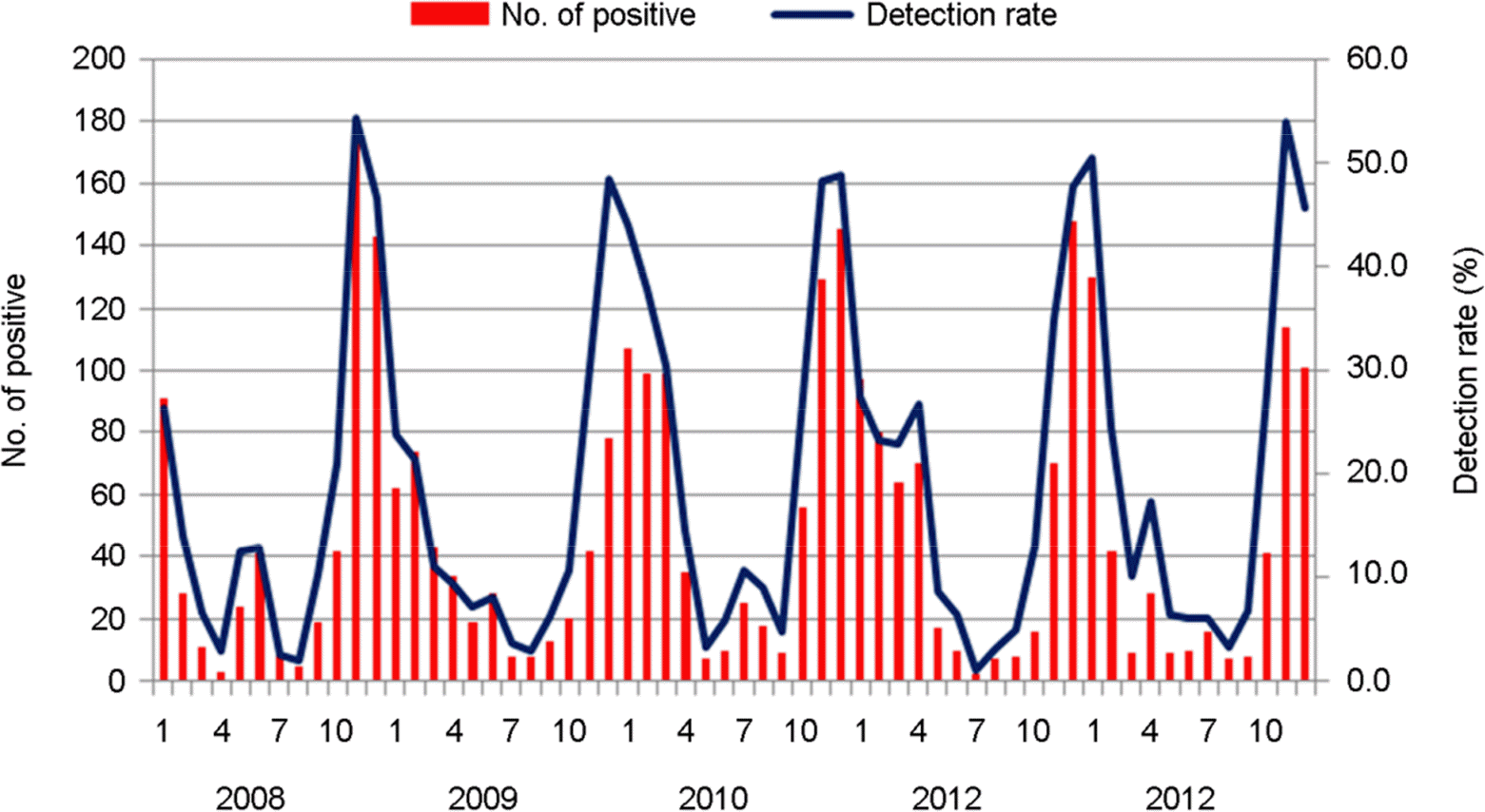 | Figure 1.Seasonal distribution of norovirus infection from diarrheal patients in Gwangju metropolitan city during 5 years (2008~2012) |
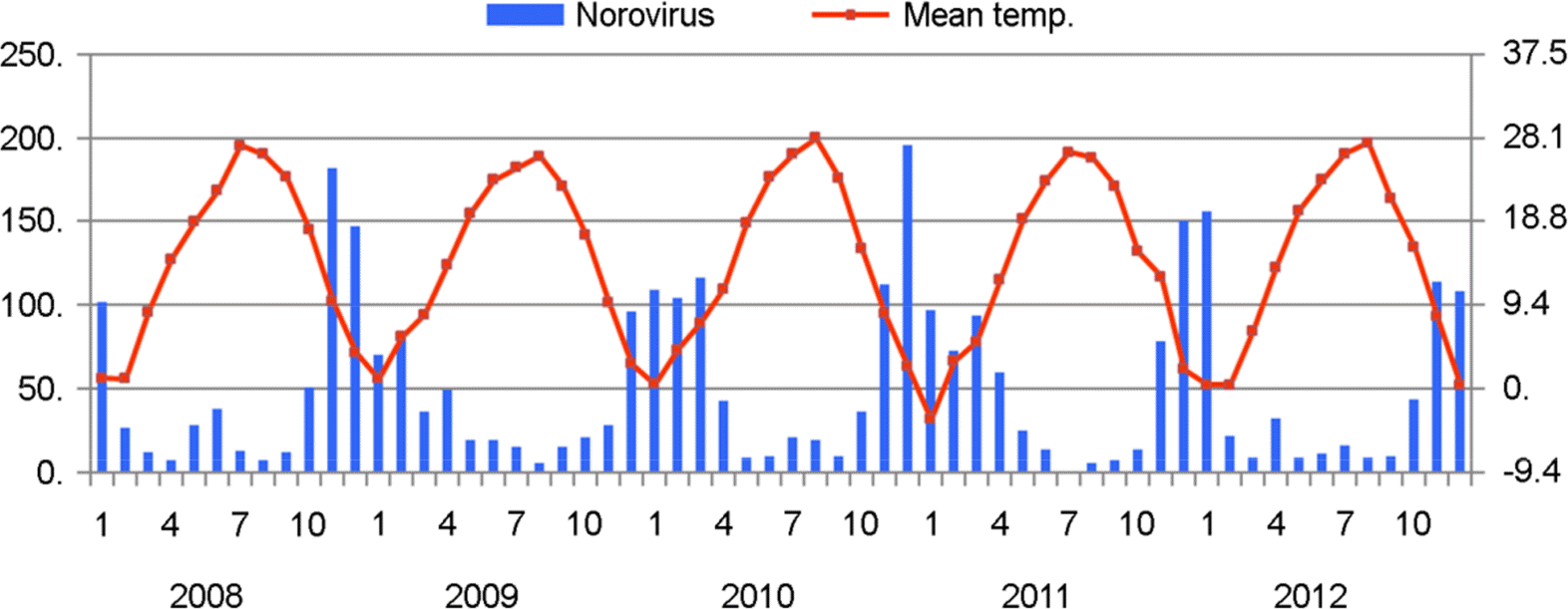 | Figure 2.Relationship between monthly distribution of norovirus from diarrheal patients and mean monthly temperature in Gwangju metropolitan city during 5 years (2008~2012) |
Table 1.
RT-PCR primers for the detection of diarrheal patients in this study
Table 2.
Prevalence of norovirus infection detected from diarrhe patients in Gwangju metropolitan city during 5 years (20082012)
| Year | No. of cases | No. of detected | Detection rate (%) |
|---|---|---|---|
| 2008 | 2,971 | 626 | 21.1 |
| 2009 | 3,147 | 455 | 14.5 |
| 2010 | 2,844 | 785 | 27.6 |
| 2011 | 2,812 | 619 | 22.0 |
| 2012 | 2,157 | 540 | 25.0 |
| Total | 13,931 | 3,025 | 21.7 |
Table 3.
Proportion of norovirus genogroup GI and GII detected in Gwangju metropolitan city during 5 years (2008~2012)
Table 4.
Annual genotypic distribution of norovirus GI in Gwangju metropolitan city during 5 years (2008~2012)
Table 5.
Annual genotypic distribution of norovirus GII in Gwangju metropolitan city during 5 years (2008~2012)
Table 6.
Change in relative frequencies of GII-4 variants in Gwangju metropolitan city during 5 years (2008~2012)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download