Abstract
Genipin, an aglycone derived from geniposide found in Gardenia jasminoides, is known to be an excellent natural cross-linker, strong apoptosis inducer, and antiviral agent. Although evidence suggests antiviral activity of genipin in several in vitro viral infection systems, there have been few literatures which review antitumor effects of genipin in a variety of in vitro/ in vivo models of cancers yet. In this review, we present some of the latest findings in the studies of genipin focusing on antitumor effects and its mechanisms. In brief, genipin inhibits mitochondrial uncoupling protein 2 to increase accumulation of reactive oxygen species, leading to ROS/c-Jun N-terminal kinase-dependent apoptosis of cancer cells. Genipin also increase tissue inhibitors of metalloproteases (MMP), resulting to decrease activities of MMP-2 which plays a key role in metastasis of cancers. Genipin has shown a biphasic effects on cell death and survival in cancer cells as many other plant-derived phytochemicals do. Finally we discuss the potential of genipin as a promosing novel antitumor agent which could be applicable to chemotherapy and/or chemoprevention for cancers.
Go to : 
REFERENCES
1). Korean Committees of Pharmacognosy Publication: Pharmacognosy. In: Committee of Pharmacognosy Publication, ed. Pharmacognosy. Volume 2. Seoul Korea: Dong Myoung. 2013; 463-4.
2). Tseng TH, Chu CY, Huang JM, Shiow SJ, Wang CJ. Crocetin protects against oxidative damage in rat primary hepatocytes. Cancer Lett. 1995; 97:61–7.

3). Ko EY, Moon A. Natural Products for Chemoprevention of Breast Cancer. J Cancer Prev. 2015; 20:223–31.

4). Djerassi C. GJD, Kincl F.A. Isolation and Characterization of Genipin. Journal of Organic Chemistry. 1960; 25:2174–7.
5). Tsai TR, Tseng TY, Chen CF, Tsai TH. Identification and determination of geniposide contained in Gardenia jasminoides and in two preparations of mixed traditional Chinese medicines. J Chromatogr A. 2002; 961:83–8.
6). Yao CH, Liu BS, Hsu SH, Chen YS, Tsai CC. Biocompatibility and biodegradation of a bone composite containing tricalcium phosphate and genipin crosslinked gelatin. J Biomed Mater Res A. 2004; 69:709–17.

7). Ayyasamy V, Owens KM, Desouki MM, Liang P, Bakin A, Thangaraj K, et al. Cellular model of Warburg effect identifies tumor promoting function of UCP2 in breast cancer and its suppression by genipin. PLoS One. 2011; 6:e24792.

8). Kim BC, Kim HG, Lee SA, Lim S, Park EH, Kim SJ, et al. Genipin-induced apoptosis in hepatoma cells is mediated by reactive oxygen species/c-Jun NH2-terminal kinase-dependent activation of mitochondrial pathway. Biochem Pharmacol. 2005; 70:1398–407.

9). Wang N, Zhu M, Tsao SW, Man K, Zhang Z, Feng Y. Up-regulation of TIMP-1 by genipin inhibits MMP-2 activities and suppresses the metastatic potential of human hepatocellular carcinoma. PLoS One. 2012; 7:e46318.

10). Hong HY, Kim BC. Mixed lineage kinase 3 connects reactive oxygen species to c-Jun NH2-terminal kinase-induced mitochondrial apoptosis in genipin-treated PC3 human prostate cancer cells. Biochem Biophys Res Commun. 2007; 362:307–12.

11). Yao ML, Gu J, Zhang YC, Wang N, Zhu ZH, Yang QT, et al. [Inhibitory effect of Genipin on uncoupling protein-2 and energy metabolism of androgen-independent prostate cancer cells]. Zhonghua Nan Ke Xue. 2015; 21:973–6.
12). Lee JH, Lee DU, Jeong CS. Gardenia jasminoides Ellis ethanol extract and its constituents reduce the risks of gastritis and reverse gastric lesions in rats. Food Chem Toxicol. 2009; 47:1127–31.

13). Ko H, Kim JM, Kim SJ, Shim SH, Ha CH, Chang HI. Induction of apoptosis by genipin inhibits cell proliferation in AGS human gastric cancer cells via Egr1/p21 signaling pathway. Bioorg Med Chem Lett. 2015; 25:4191–6.

14). Son M, Lee M, Ryu E, Moon A, Jeong CS, Jung YW, et al. Genipin as a novel chemical activator of EBV lytic cycle. J Microbiol. 2015; 53:155–65.

15). Kim JM, Ko H, Kim SJ, Shim SH, Ha CH, Chang HI. Chemopreventive Properties of Genipin on AGS Cell Line via Induction of JNK/Nrf2/ARE Signaling Pathway. J Biochem Mol Toxicol. 2016; 30:45–54.

16). Cao H, Feng Q, Xu W, Li X, Kang Z, Ren Y, et al. Genipin induced apoptosis associated with activation of the c-Jun NH2-terminal kinase and p53 protein in HeLa cells. Biol Pharm Bull. 2010; 33:1343–8.

17). Mailloux RJ, Adjeitey CN, Harper ME. Genipin-induced inhibition of uncoupling protein-2 sensitizes drug-resistant cancer cells to cytotoxic agents. PLoS One. 2010; 5:e13289.

18). Feng Q, Cao HL, Xu W, Li XR, Ren YQ, Du LF. Apoptosis induced by genipin in human leukemia K562 cells: involvement of c-Jun N-terminal kinase in G(2)/M arrest. Acta Pharmacol Sin. 2011; 32:519–27.

19). Lee JC, Ahn KS, Jeong SJ, Jung JH, Kwon TR, Rhee YH, et al. Signal transducer and activator of transcription 3 pathway mediates genipin-induced apoptosis in U266 multiple myeloma cells. J Cell Biochem. 2011; 112:1552–62.

20). Kim ES, Jeong CS, Moon A. Genipin, a constituent of Gardenia jasminoides Ellis, induces apoptosis and inhibits invasion in MDA-MB-231 breast cancer cells. Oncol Rep. 2012; 27:567–72.
21). Pons DG, Nadal-Serrano M, Torrens-Mas M, Valle A, Oliver J, Roca P. UCP2 inhibition sensitizes breast cancer cells to therapeutic agents by increasing oxidative stress. Free Radic Biol Med. 2015; 86:67–77.

22). Yang X, Yao J, Luo Y, Han Y, Wang Z, Du L. P38 MAP kinase mediates apoptosis after genipin treatment in non-small-cell lung cancer H1299 cells via a mitochondrial apoptotic cascade. J Pharmacol Sci. 2013; 121:272–81.
23). Suzuki Y, Kondo K, Ikeda Y, Umemura K. Antithrombotic effect of geniposide and genipin in the mouse thrombosis model. Planta Med. 2001; 67:807–10.

Go to : 
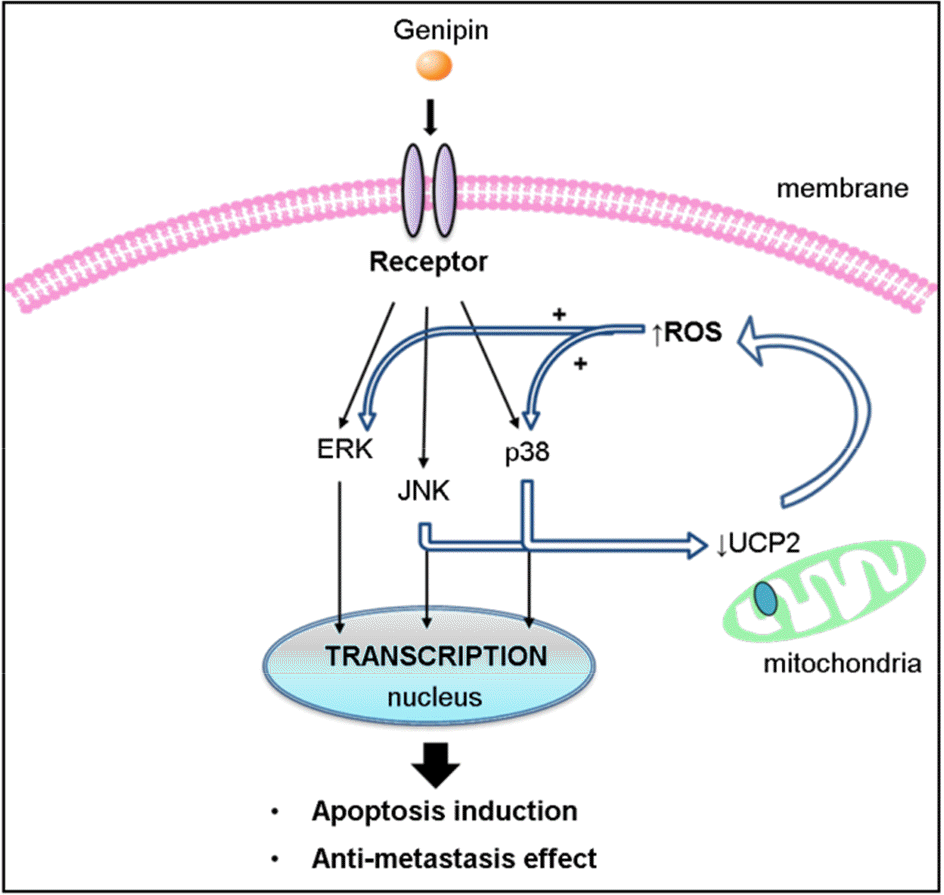 | Figure 1.
Model for the role of UCP2 in genipin-induced mitochondrial ROS signaling. Recognition of genipin by receptor activates JNK, p38 and ERK signaling pathways. Meanwhile, a ROS signaling pathway is set up. UCP2 is quickly down-regulated in response to genipin via the JNK and p38 pathways to increase mitochondrial ROS production. In this way, mitochondria contribute to the apoptosis induction and anti-metastasis effect. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download