Abstract
Streptococcus pneumoniae (S. pneumoniae, also known as pneumococcus) infections are major causes of death worldwide. Despite the development and use of effective antibiotics, high, early mortality due to pneumococcal infections has not been decreased for the last few decades. Recent study found a deadly hemorrhagic acute lung injury (ALI) as a major cause of death at the early stage of severe pneumococcal infections. Interleukin (IL)-1β was known to play critical roles not only for the development of ALI but also resolution of it. The role of IL-1β on the pathogenesis of pneumococcal ALI, however, has not been well understood yet. This study aims to investigate the role of IL-1β on the development of pneumococcal ALI and subsequent death. IL-1β expression was upregulated in the lungs of pneumococcal ALI in wild-type (WT) mice, but not in the plasma. Despite an increased expression of pulmonary IL-1β, no inflammatory cell infiltration into airway has been observed. Upregulation of IL-1β expression was indeed dependent on pneumococcal cytoplasmic toxin pneumolysin and its cell surface receptor Toll-like receptor 4. Deficiency of IL-1R1, a cell surface receptor of IL-1β, resulted in a markedly reduced hemorrhagic pulmonary edema and early death in pneumococcal ALI. Finally, IL-1β neutralization in WT mice protects against pulmonary hemorrhagic edema and death. These data suggest that pulmonary expression of IL-1β exacerbates pneumolysin-induced ALI and death by promoting alveolar hemorrhagic edema.
Go to : 
REFERENCES
1). Grigoryev DN, Finigan JH, Hassoun P, Garcia JG. Science review: searching for gene candidates in acute lung injury. Crit Care. 2004; 8:440–7.
2). Happel KI, Nelson S, Summer W. The lung in sepsis: fueling the fire. Am J Med Sci. 2004; 328:230–7.

3). Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol. 2011; 6:147–63.

4). Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012; 122:2731–40.

5). Schwarz MA. Acute lung injury: cellular mechanisms and derangements. Paediatr Respir Rev. 2001; 2:3–9.

6). Brandenburg JA, Marrie TJ, Coley CM, Singer DE, Obrosky DS, Kapoor WN, et al. Clinical presentation, processes and outcomes of care for patients with pneumococcal pneumonia. J Gen Intern Med. 2000; 15:638–46.

7). Hollingshead SK, Briles DE. Streptococcus pneumoniae: new tools for an old pathogen. Curr Opin Microbiol. 2001; 4:71–7.
8). Marriott HM, Mitchell TJ, Dockrell DH. Pneumolysin: a double-edged sword during the host-pathogen interaction. Curr Mol Med. 2008; 8:497–509.

9). Wood WB. Studies on the mechanism of recovery in pneumococcal pneumonia: I. The action of type specific antibody upon the pulmonary lesion of experimental pneumonia. J Exp Med. 1941; 73:201–22.
10). Chavanet P. Pneumococcus infections: is the burden still as heavy? Med Mal Infect. 2012; 42:149–53.

11). Feldman C, Anderson R. Recent advances in our understanding of Streptococcus pneumoniae infection. F1000- Prime Rep. 2014; 6:82.

12). Shin SG, Koh SH, Lim JH. The in vivo and in vitro roles of epithelial pattern recognition receptors in pneumococcal infections. J Bacteriol Virol. 2014; 44:121–32.
13). World Health Organization. Pneumococcal conjugate vaccine for childhood immunization: WHO position paper. Wkly Epidemiol Rec. 2007; 82:93–104.
14). Maus UA, Srivastava M, Paton JC, Mack M, Everhart MB, Blackwell TS, et al. Pneumolysin-induced lung injury is independent of leukocyte trafficking into the alveolar space. J Immunol. 2004; 173:1307–12.

15). Lim JH, Stirling B, Derry J, Koga T, Jono H, Woo CH, et al. Tumor suppressor CYLD regulates acute lung injury in lethal Streptococcus pneumoniae infections. Immunity. 2007; 27:349–60.
16). Cockeran R, Anderson R, Feldman C. The role of pneumolysin in the pathogenesis of Streptococcus pneumoniae infection. Curr Opin Infect Dis. 2002; 15:235–9.
17). Mitchell TJ, Dalziel CE. The biology of pneumolysin. Subcell Biochem. 2014; 80:145–60.
18). Rubins JB, Duane PG, Clawson D, Charboneau D, Young J, Niewoehner DE. Toxicity of pneumolysin to pulmonary alveolar epithelial cells. Infect Immun. 1993; 61:1352–8.

19). Lim JH, Jono H, Koga T, Woo CH, Ishinaga H, Bourne P, et al. Tumor suppressor CYLD acts as a negative regulator for non-typeable Haemophilus influenza-induced inflammation in the middle ear and lung of mice. PLoS One. 2007; 2:e1032.

20). Lim JH, Ha UH, Woo CH, Xu H, Li JD. CYLD is a crucial negative regulator of innate immune response in Escherichia coli pneumonia. Cell Microbiol. 2008; 10:2247–56.
21). Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002; 13:323–40.

22). Lim JH, Ha U, Sakai A, Woo CH, Kweon SM, Xu H, et al. Streptococcus pneumoniae synergizes with nontypeable Haemophilus influenzae to induce inflammation via upregulating TLR2. BMC Immunol. 2008; 9:40.
23). Ishinaga H, Jono H, Lim JH, Komatsu K, Xu X, Lee J, et al. Synergistic induction of nuclear factor-kappaB by transforming growth factor-beta and tumour necrosis factor-alpha is mediated by protein kinase A-dependent RelA acetylation. Biochem J. 2009; 417:583–91.
24). Wu J, Yan Z, Schwartz DE, Yu J, Malik AB, Hu G. Activation of NLRP3 inflammasome in alveolar macrophages contributes to mechanical stretch-induced lung inflammation and injury. J Immunol. 2013; 190:3590–9.

25). Kolb M, Margetts PJ, Anthony DC, Pitossi F, Gauldie J. Transient expression of IL-1beta induces acute lung injury and chronic repair leading to pulmonary fibrosis. J Clin Invest. 2001; 107:1529–36.
26). Gasse P, Mary C, Guenon I, Noulin N, Charron S, Schnyder-Candrian S, et al. IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. J Clin Invest. 2007; 117:3786–99.

27). Frank JA, Pittet JF, Wray C, Matthay MA. Protection from experimental ventilator-induced acute lung injury by IL-1 receptor blockade. Thorax. 2008; 63:147–53.

28). Ganter MT, Roux J, Miyazawa B, Howard M, Frank JA, Su G, et al. Interleukin-1beta causes acute lung injury via alphavbeta5 and alphavbeta6 integrin-dependent mechanisms. Circ Res. 2008; 102:804–12.
29). Berry AM, Yother J, Briles DE, Hansman D, Paton JC. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect Immun. 1989; 57:2037–42.
30). Malley R, Henneke P, Morse SC, Cieslewicz MJ, Lipsitch M, Thompson CM, et al. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc Natl Acad Sci U S A. 2003; 100:1966–71.

31). Lim JH, Jono H, Komatsu K, Woo CH, Lee J, Miyata M, et al. CYLD negatively regulates transforming growth factor-β-signalling via deubiquitinating Akt. Nat Commun. 2012; 3:771.

32). Lim JH, Kim HJ, Komatsu K, Ha U, Huang Y, Jono H, et al. Differential regulation of Streptococcus pneumoniae-induced human MUC5AC mucin expression through distinct MAPK pathways. Am J Transl Res. 2009; 1:300–11.
33). Woo CH, Shin SG, Koh SH, Lim JH. TBX21 participates in innate immune response by regulating Toll-like receptor 2 expression in Streptococcus pneumoniae infections. Mol Oral Microbiol. 2014; 29:233–43.
34). Srivastava A, Henneke P, Visintin A, Morse SC, Martin V, Watkins C, et al. The apoptotic response to pneumolysin is Toll-like receptor 4 dependent and protects against pneumococcal disease. Infect Immun. 2005; 73:6479–87.

35). Hwang MW, Matsumori A, Furukawa Y, Ono K, Okada M, Iwasaki A, et al. Neutralization of interleukin-1beta in the acute phase of myocardial infarction promotes the progression of left ventricular remodeling. J Am Coll Cardiol. 2001; 38:1546–53.
36). Cho A, Seok SH. Ethical guidelines for use of experimental animals in biomedical research. J Bacteriol Virol. 2013; 43:18–26.

37). Wong CH, Song C, Heng KS, Kee IH, Tien SL, Kumarasinghe P, et al. Plasma free hemoglobin: a novel diagnostic test for assessment of the depth of burn injury. Plast Reconstr Surg. 2006; 117:1206–13.

38). Folz RJ, Abushamaa AM, Suliman HB. Extracellular superoxide dismutase in the airways of transgenic mice reduces inflammation and attenuates lung toxicity following hyperoxia. J Clin Invest. 1999; 103:1055–66.

39). Lim JH, Woo CH, Li JD. Critical role of type 1 plasminogen activator inhibitor (PAI-1) in early host defense against nontypeable Haemophilus influenzae (NTHi) infection. Biochem Biophys Res Commun. 2011; 414:67–72.

40). Shin SG, Koh SH, Woo CH, Lim JH. PAI-1 inhibits development of chronic otitis media and tympanosclerosis in a mouse model of otitis media. Acta Otolaryngol. 2014; 134:1231–8.

41). Rijneveld AW, Florquin S, Speelman P, Edwards CK, Dinarello CA, van der Poll T. Interleukin-1 receptor antagonist transiently impairs antibacterial defense but not survival in murine pneumococcal pneumonia. Eur Cytokine Netw. 2003; 14:242–5.
42). Kafka D, Ling E, Feldman G, Benharroch D, Voronov E, Givon-Lavi N, et al. Contribution of IL-1 to resistance to Streptococcus pneumoniae infection. Int Immunol. 2008; 20:1139–46.
43). Dinarello CA. Overview of the interleukin-1 family of ligands and receptors. Semin Immunol. 2013; 25:389–93.

44). Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013; 39:1003–18.

45). Park HK, Woo SY. Detection of Streptococcus pneumoniae pneumolysin gene by PCR in sera and cerebrospinal fluids from hospitalized patients. J Bacteriol Virol. 2001; 31:307–16.
46). Geiser T. Mechanisms of alveolar epithelial repair in acute lung injury-a translational approach. Swiss Med Wkly. 2003; 133:586–90.
47). Marriott HM, Gascoyne KA, Gowda R, Geary I, Nicklin MJ, Iannelli F, et al. Interleukin-1β regulates CXCL8 release and influences disease outcome in response to Streptococcus pneumoniae, defining intercellular cooperation between pulmonary epithelial cells and macrophages. Infect Immun. 2012; 80:1140–9.
48). Dockrell DH, Lee M, Lynch DH, Read RC. Immune-mediated phagocytosis and killing of Streptococcus pneumoniae are associated with direct and bystander macrophage apoptosis. J Infect Dis. 2001; 184:713–22.
49). Ali F, Lee ME, Iannelli F, Pozzi G, Mitchell TJ, Read RC, et al. Streptococcus pneumoniae-associated human macrophage apoptosis after bacterial internalization via complement and Fcgamma receptors correlates with intracellular bacterial load. J Infect Dis. 2003; 188:1119–31.
50). Bewley MA, Naughton M, Preston J, Mitchell A, Holmes A, Marriott HM, et al. Pneumolysin activates macrophage lysosomal membrane permeabilization and executes apoptosis by distinct mechanisms without membrane pore formation. MBio. 2014; 5:e01710–14.

51). Pugin J, Ricou B, Steinberg KP, Suter PM, Martin TR. Proinflammatory activity in bronchoalveolar lavage fluids from patients with ARDS, a prominent role for interleukin-1. Am J Respir Crit Care Med. 1996; 153:1850–6.

52). Pugin J, Verghese G, Widmer MC, Matthay MA. The alveolar space is the site of intense inflammatory and profibrotic reactions in the early phase of acute respiratory distress syndrome. Crit Care Med. 1999; 27:304–12.

53). Olman MA, White KE, Ware LB, Cross MT, Zhu S, Matthay MA. Microarray analysis indicates that pulmonary edema fluid from patients with acute lung injury mediates inflammation, mitogen gene expression, and fibroblast proliferation through bioactive interleukin-1. Chest. 2002; 121:69S–70S.

54). Geiser T, Atabai K, Jarreau PH, Ware LB, Pugin J, Matthay MA. Pulmonary edema fluid from patients with acute lung injury augments in vitro alveolar epithelial repair by an IL-1beta-dependent mechanism. Am J Respir Crit Care Med. 2001; 163:1384–8.
55). Geiser T, Jarreau PH, Atabai K, Matthay MA. Interleukin-1beta augments in vitro alveolar epithelial repair. Am J Physiol Lung Cell Mol Physiol. 2000; 279:L1184–90.
Go to : 
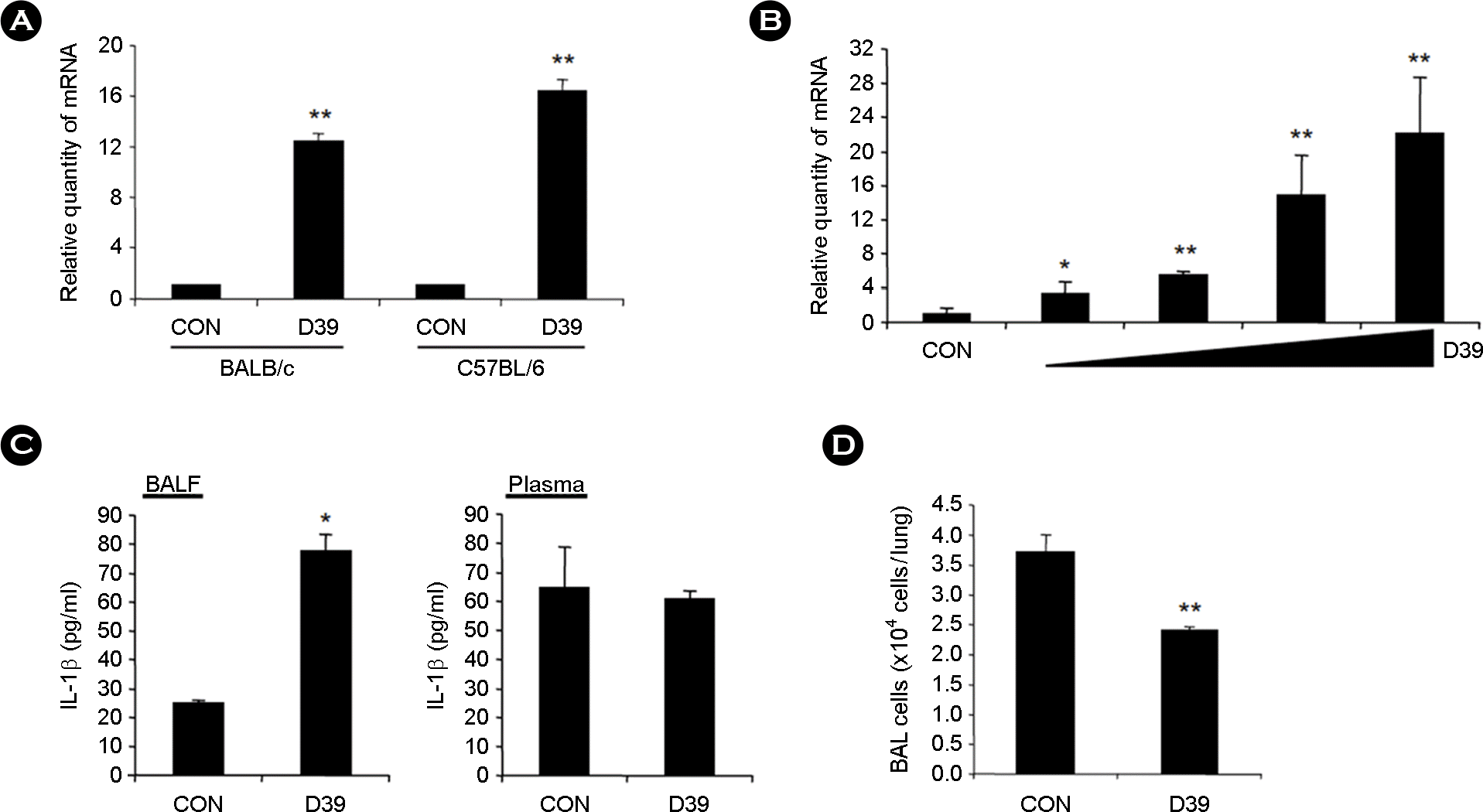 | Figure 1.
IL-1β expression is upregulated in the lungs of pneumococcal ALI, but not in the plasma. A, BALB/c and C57BL/6 mice were intratracheally (i.t.) inoculated with pneumococcal D39 lysate equivalent to the 5 × 107 colony forming unit (CFU), and mRNA expression of IL-1β was measured from the lungs of mice 3 hours after i.t. inoculation by real-time quantitative PCR (Q-PCR) assay. B, C57BL/6 mice were i.t. inoculated with various amount of pneumococcal D39 lysate (equivalent to 0.625 ~ 5 × 107 CFU), and mRNA expression of IL-1β was measured from the lungs of mice 3 hours after i.t. inoculation by Q-PCR assay. C, BALB/c mice were i.t. inoculated with pneumococcal D39 lysate, and protein expression of IL-1β was measured from the BALF and plasma of mice 3 hours after i.t. inoculation by ELISA assay. D, C57BL/6 mice were i.t. inoculated with pneumococcal D39 lysate, and bronchoalveolar lavage (BAL) was conducted 3 hours after i.t. inoculation. Cell numbers in the BAL fluid (BALF) were counted. Data are the means ± SD. ∗, p <0.05 compared with CON; ∗∗, p < 0.01 compared with CON. CON, control. |
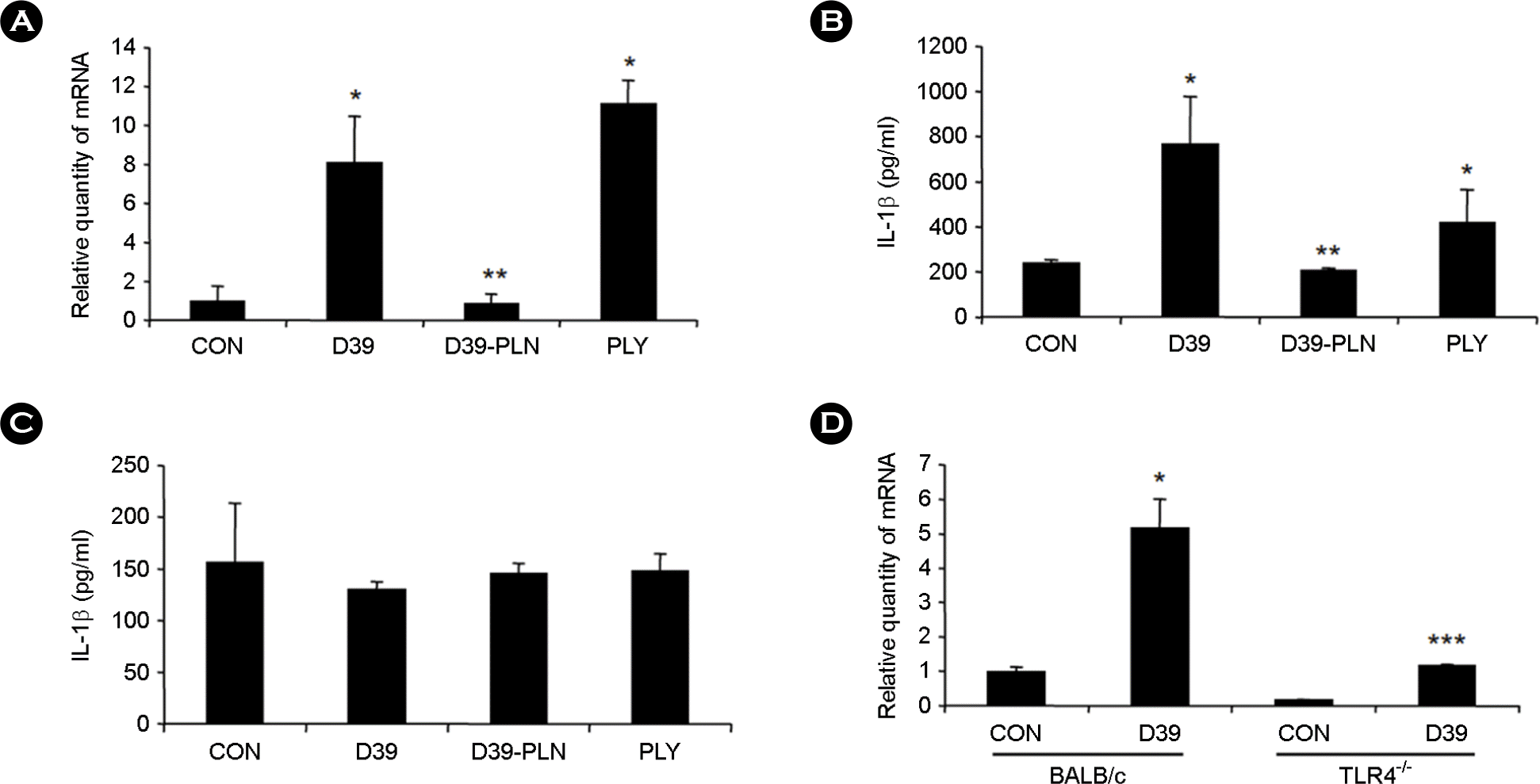 | Figure 2.
Pneumococcal pneumolysin-induced upregulation of pulmonary IL-1β is dependent on a cellular TLR4. A, C57BL/6 mice were i.t. inoculated with pneumococcal D39 lysate, D39-PLN, or pneumolysin (PLY), and mRNA expression of IL-1β was measured from the lungs of mice 3 hours after i.t. inoculation by Q-PCR assay. B & C, C57BL/6 mice were i.t. inoculated with pneumococcal D39 lysate, D39-PLN, or PLY, and protein expression of IL-1β was measured from the BALF (B) and plasma (C) of mice 3 hours after i.t. inoculation by ELISA assay. D, TLR4-/- mice were i.t. inoculated with pneumococcal D39 lysate, and expression levels of IL-1β mRNA were compared to those of genetic background control BALB/c mice by Q-PCR assay. Data are the means ± SD. ∗, p < 0.01 compared with CON; ∗∗, p < 0.05 compared with D39 (A-C); ∗∗∗, p < 0.05 compared with D39 BALB/c mice. CON, control; PLY, pneumolysin. |
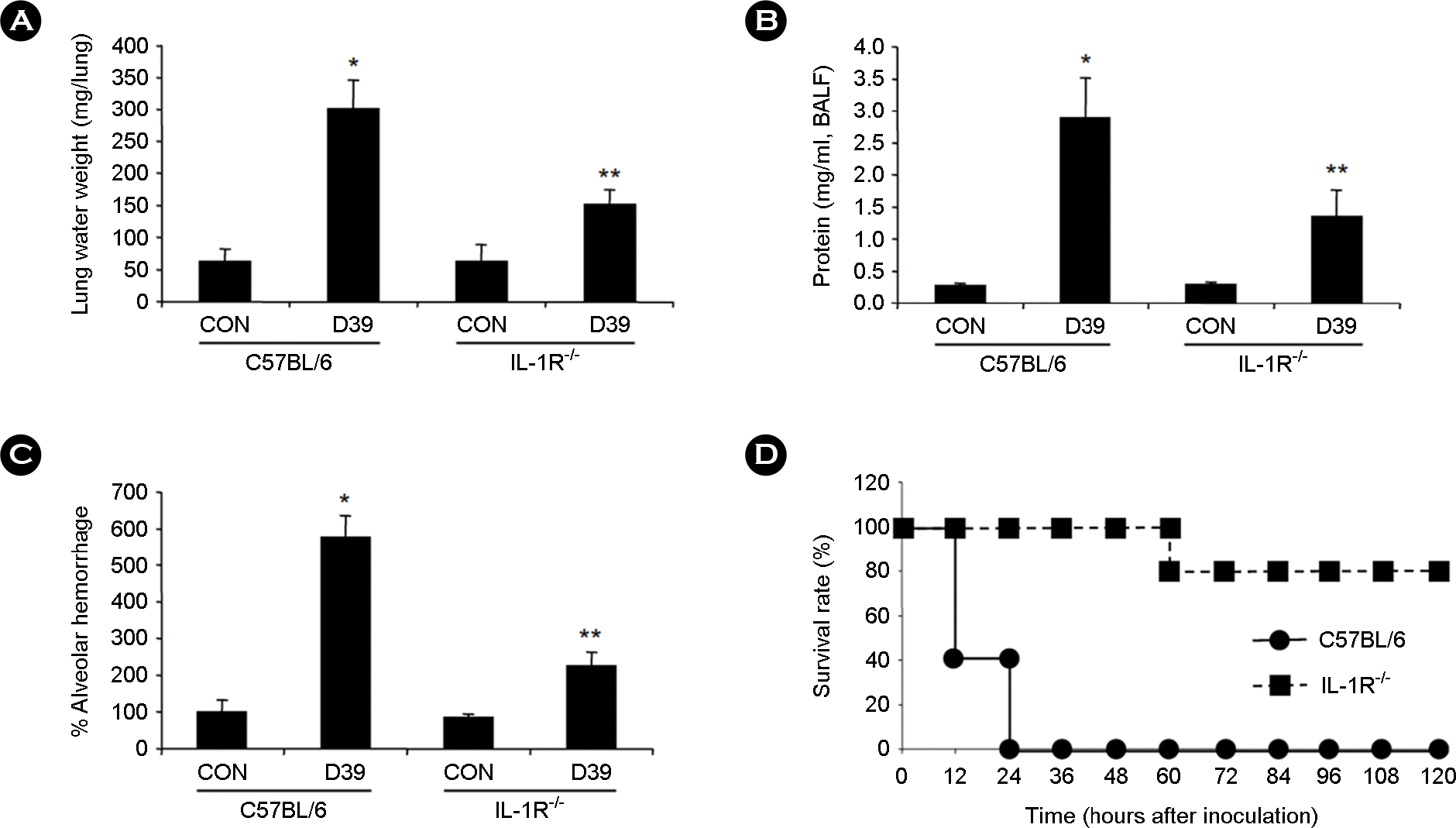 | Figure 3.
Deficiency of IL-1R1 protects mice against pneumococcal ALI and death. A-C, IL-1R1-/- and control C57BL/6 mice were i.t. inoculated with pneumococcal D39 lysate, and lung extravascular water weight (A), alveolar protein permeability (B), and alveolar hemoglobin (C) were measured from the lungs of mice 3 hours after i.t. inoculation. D, IL-1R1-/- and control C57BL/6 mice were i.t. inoculated with pneumococcal D39 lysate, and survival rates were recorded for 5 days. Viability in D was assessed by using Kaplan-Meier survival analysis and compared by long-lank test. Data are the means ± SD. ∗, p < 0.01 compared with CON in C57BL/6; ∗∗, p < 0.05 compared with D39 in C57BL/6 mice. CON, control. |
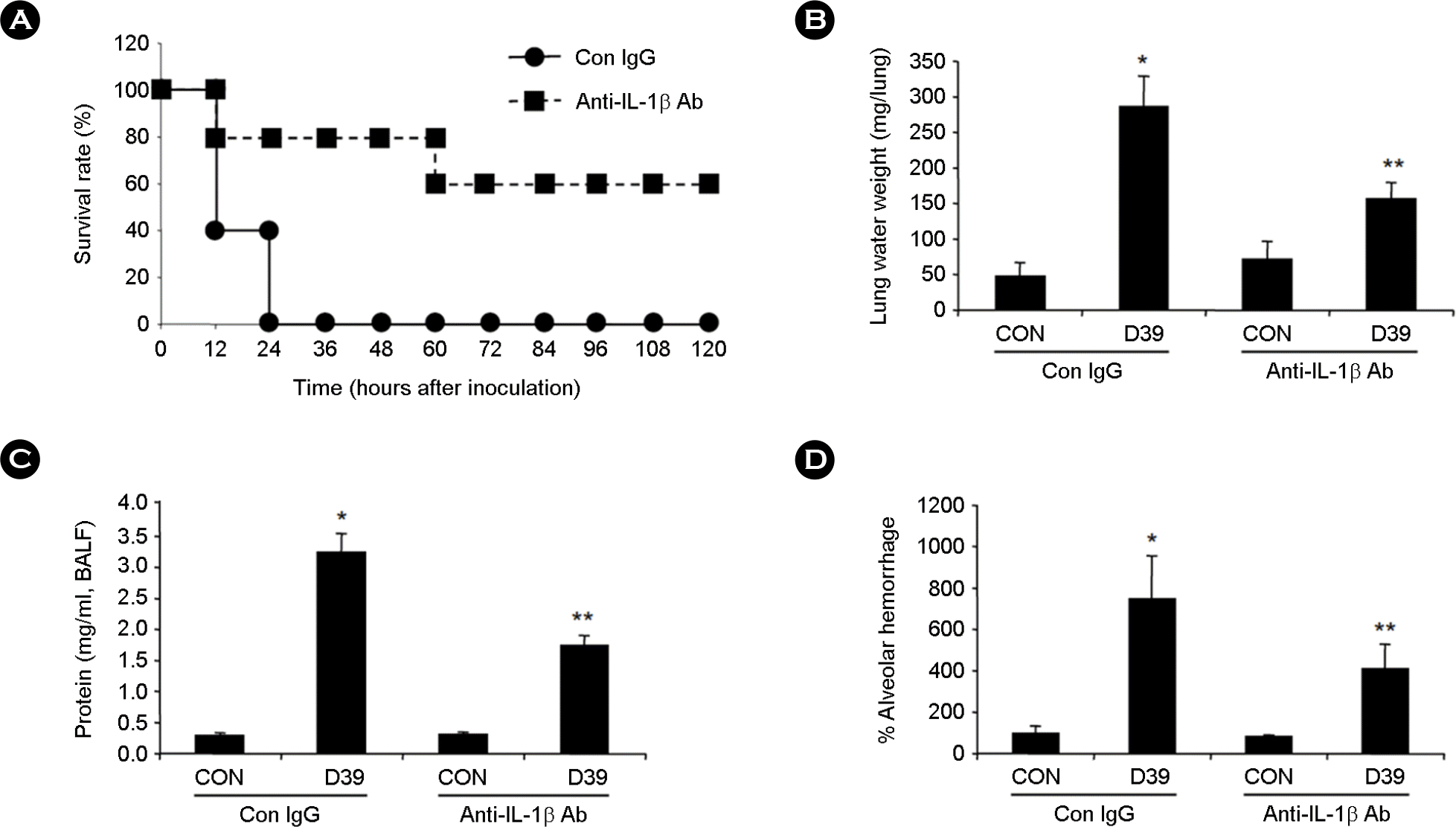 | Figure 4.
Pulmonary neutralization of IL-1β inhibited pneumococcal ALI and death in WT mice. A, C57BL/6 mice were i.t. inoculated with 100 μg of anti-mouse IL-1β antibody or control IgG, and pneumococcal D39 lysate were i.t. inoculated 2 hours after antibody inoculation. Survival rates were recorded for 5 days, and viability was assessed by using Kaplan-Meier survival analysis. B-D, C57BL/6 mice were i.t. inoculated with 100 μg of anti-mouse IL-1β antibody for 2 hours followed by i.t. inoculation of pneumococcal D39 lysate. Lung extravascular water weight (B), alveolar protein permeability (C), and alveolar hemoglobin (D) were measured from the lungs of mice 3 hours after i.t. inoculation of D39. Data in B – D are the means ± SD. ∗, p < 0.01 compared with CON in WT mice treated with Con IgG; ∗∗, p < 0.05 compared with D39 in WT mice treated with Con IgG. CON, control. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download