Abstract
Continuous increase of bacterial resistance to antibiotics causes many problems such as the advent of resistance to pathogenic bacteria, difficulty of microbial disease treatments, environmental pollution and others. It is inevitable to find potential substitutes for antibiotics in order to solve the above mentioned problems. Recently many literatures have shown that probiotics could be applied to the treatment or amelioration of respiratory diseases in addition to intensively studied gut related diseases. Target diseases for collecting data and analysis of the efficacies were chosen because viral respiratory infections are the most common diseases in humans. They were mainly viral diseases like common colds, pneumonia in addition to allergies and asthma. Papers on clinical efficacies, safety risks and mechanisms of microbial action of respiratory diseases were secured through known information sites and analyzed for their exact evaluations. The present analysis of research results on probiotics efficacies for respiratory diseases showed discrepancies in efficacies. On the whole, half to one third of papers reviewed only showed certain level of efficacies against respiratory viral diseases. It is very difficult to compare the results directly because the studies varied highly in study design, outcome measures, probiotics, dose, and matrices used. However, the results obtained so far show the potential applications of probiotics to the prevention or amelioration of the diseases. Conclusively, further well organized studies using randomized, double-blind, placebo-controlled clinical trials are needed to elucidate the realities of probiotics on respiratory related diseases and to obtain more definite efficacy results.
REFERENCES
1). Goossens H, Ferech M, Vander Stichele R, Elseviers M. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005; 365:579–87.

2). Hurd HS, Doores S, Hayes D, Mathew A, Maurer J, Silley P, et al. Public health consequences of macrolide use in food animals: a deterministic risk assessment. J Food Prot. 2004; 67:980–92.

3). Filteau M, Matamoros S, Savard P, Roy D. Molecular monitoring of fecal microbiota in healthy adults following probiotic yogurt intake. 2013; 1:123–9.
4). Kumar M, Mohania D, Poddar D, Behare PV, Nagpal R, Kumar A, et al. A probiotic fermented milk prepared by mixed culture combination reduces pathogen shedding and alleviates disease signs in rats challenged with pathogens. Int J Probiotics Prebiotics. 2009; 4:211–8.
5). Kumar M, Kumar A, Nagpal R, Mohania D, Behare P, Verma V, et al. Cancer-preventing attributes of probiotics: an update. Int J Food Sci Nutr. 2010; 61:473–96.

6). Kumar M, Verma V, Nagpal R, Kumar A, Behare PV, Singh B, et al. Anticarcinogenic effect of probiotic fermented milk and chlorophyllin on aflatoxin-B1-induced liver carcinogenesis in rats. Br J Nutr. 2012; 107:1006–16.
7). Oelschlaeger TA. Mechanisms of probiotic actions – A review. Int J Med Microbiol. 2010; 300:57–62.

8). Reid G, Friendship R. Alternatives to antibiotic use: probiotics for the gut. Anim Biotechnol. 2002; 13:97–112.

9). Schlundt J. Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria. Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. FAO/WHO;2012.
10). Araya M, Morelli L, Reid G, Sanders ME, Stanton C, Pineiro M, et al. Guidelines for the evaluation of probiotics in food. FAO/WHO Work Group Rep;2002. p. 1–11.
11). Sze MA, Dimitriu PA, Hayashi S, Elliott WM, McDonough JE, Gosselink JV, et al. The lung tissue microbiome in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012; 185:1073–80.

12). Schlee M, Wehkamp J, Altenhoefer A, Oelschlaeger TA, Stange EF, Fellermann K. Induction of human beta-defensin 2 by the probiotic Escherichia coli Nissle 1917 is mediated through flagellin. Infect Immun. 2007; 75:2399–407.
13). Gollwitzer ES, Marsland BJ. Microbiota abnormalities in inflammatory airway diseases – Potential for therapy. Pharmacol Ther. 2014; 141:32–9.

14). Charlson ES, Bittinger K, Haas AR, Fitzgerald AS, Frank I, Yadav A, et al. Topographical continuity of bacterial populations in the healthy human respiratory tract. Am J Respir Crit Care Med. 2011; 184:957–63.

15). Alexandre Y, Le Blay G, Boisramé-Gastrin S, Le Gall F, Héry-Arnaud G, Gouriou S, et al. Probiotics: A new way to fight bacterial pulmonary infections? Med Mal Infect. 2014; 44:9–17.

16). Bousbia S, Papazian L, Saux P, Forel JM, Auffray JP, Martin C, et al. Repertoire of intensive care unit pneumonia microbiota. PloS One. 2012; 7:e32486.

17). Bosch M, Méndez M, Pérez M, Farran A, Fuentes MC, Cuñé J. Lactobacillus plantarum CECT7315 and CECT- 7316 stimulate immunoglobulin production after influenza vaccination in elderly. Nutr Hosp. 2012; 27:504–9.
18). Jones ML, Tomaro-Duchesneau C, Prakash S. The gut microbiome, probiotics, bile acids axis, and human health. Trends Microbiol. 2014; 22:306–8.

19). Reid G, Jass J, Sebulsky MT, McCormick JK. Potential uses of probiotics in clinical practice. Clin Microbiol Rev. 2003; 16:658–72.

20). de Vrese M, Marteau PR. Probiotics and prebiotics: effects on diarrhea. J Nutr. 2007; 137:803S–11S.

21). Falagas M, Betsi GI, Athanasiou S. Probiotics for the treatment of women with bacterial vaginosis. Clin Microbiol Infect. 2007; 13:657–64.

22). Hao Q, Lu Z, Dong BR, Huang CQ, Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst Rev. 2011; 7:CD006895.

23). Popova M, Molimard P, Courau S, Crociani D, Dufour C, Le Vacon F, et al. Beneficial effects of probiotics in upper respiratory tract infections and their mechanical actions to antagonize pathogens. J Appl Microbiol. 2012; 113:1305–18.

24). Wolvers D, Antoine JM, Myllyluoma E, Schrezenmeir J, Szajewska H, Rijkers GT. Guidance for substantiating the evidence for beneficial effects of probiotics: prevention and management of infections by probiotics. J Nutr. 2010; 140:698S–712S.

25). Siempos II, Ntaidou TK, Falagas ME. Impact of the administration of probiotics on the incidence of ventilator-associated pneumonia: a meta-analysis of randomized controlled trials. Crit Care Med. 2010; 38:954–62.

26). Cohen R, Martin E, de La Rocque F, Thollot F, Pecquet S, Werner A, et al. Probiotics and prebiotics in preventing episodes of acute otitis media in high-risk children: a randomized, double-blind, placebo-controlled study. Pediatr Infect Dis J. 2013; 32:810–4.
27). West NP, Horn PL, Pyne DB, Gebski VJ, Lahtinen SJ, Fricker PA, et al. Probiotic supplementation for respiratory and gastrointestinal illness symptoms in healthy physically active individuals. Clin Nutr. 2014; 33:581–7.

28). Kopp MV, Ankermann T, Härtel C. Clinical potential for the use of probiotics in the management of respiratory conditions and coldand influenza-like symptoms. Nutrition and Dietary Supplements. 2011; 3:51–8.
29). Luoto R, Ruuskanen O, Waris M, Kalliomäki M, Salminen S, Isolauri E. Prebiotic and probiotic supplementation prevents rhinovirus infections in preterm infants: a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2014; 133:405–13.
30). Homayouni Rad A, Torab R, Mortazavian AM, Mehrabany EV, Mehrabany LV. Can probiotics prevent or improve common cold and influenza? Nutrition. 2013; 29:805–6.

32). de Vrese M, Schrezenmeir J. Probiotics and non-intestinal infectious conditions. Br J Nutr. 2002; 88:S59–66.

33). Hatakka K, Saxelin M. Probiotics in intestinal and non-intestinal infectious diseases–Clinical evidence. Curr Pharm Des. 2008; 14:1351–67.
34). Rautava S, Salminen S, Isolauri E. Specific probiotics in reducing the risk of acute infections in infancy–a randomised, double-blind, placebo-controlled study. Br J Nutr. 2009; 101:1722–6.
35). Leyer GL, Li S, Mubasher ME, Reifer C, Ouwehand AC. Probiotic effects on cold and influenza-like symptom incidence and duration in children. Pediatrics. 2009; 124:e172–9.

36). Kukkonen K, Savilahti E, Haahtela T, Juntunen-Backman K, Korpela R, Poussa T, et al. Long-term safety and impact on infection rates of postnatal probiotic and prebiotic (synbiotic) treatment: Randomized, double-blind, placebo-controlled trial. Pediatrics. 2008; 122:8–12.

37). Aubin J, Remigy M, Verseil L, Bourdet-Sicard R, Vaudaine S, van derWerf S, et al. A probiotic fermented dairy product improves immune response to influenza vaccination in the elderly. Proceedings of the Nutrition Society. 2008; 67:E205.

38). Boge T, Rémigy M, Vaudaine S, Tanguy J, Bourdet-Sicard R, van der Werf S. A probiotic fermented dairy drink improves antibody response to influenza vaccination in the elderly in two randomised controlled trials. Vaccine. 2009; 27:5677–84.

39). Davidson LE, Fiorino AM, Snydman DR, Hibberd PL. Lactobacillus GG as an immune adjuvant for live-attenuated influenza vaccine in healthy adults: A randomized double-blind placebo-controlled trial. Eur J Clin Nutr. 2011; 65:501–7.
40). Namba K, Hatano M, Yaeshima T, Takase M, Suzuki K. Effects of Bifidobacterium longum BB536 administration on influenza infection, influenza vaccine antibody titer, and cell-mediated immunity in the elderly. Biosci Biotechnol Biochem. 2010; 74:939–45.
41). de Vrese M, Winkler P, Rautenberg P, Harder T, Noah C, Laue C, et al. Effect of Lactobacillus gasseri PA 16/8, Bifidobacterium longum SP 07/3, B. bifidum MF 20/5 on common cold episodes: A double blind, randomized, controlled trial. Clin Nutr. 2005; 24:481–91.

42). Morrow LE, Kollef MH, Casale TB. Probiotic prophylaxis of ventilator-associated pneumonia: a blinded, randomized, controlled trial. Am J Respir Crit Care Med. 2010; 182:1058–64.
43). Forestier C, Guelon D, Cluytens V, Gillart T, Sirot J, De Champs C. Oral probiotic and prevention of Pseudomonas aeruginosa infections: a randomized, double-blind, placebo-controlled pilot study in intensive care unit patients. Crit Care. 2008; 12:R69.
44). Klarin B, Molin G, Jeppsson B, Larsson A. Use of the probiotic Lactobacillus plantarum 299 to reduce pathogenic bacteria in the oropharynx of intubated patients: a randomised controlled open pilot study. Crit Care. 2008; 12:R136.
45). Knight DJ, Gardiner D, Banks A, Snape SE, Weston VC, Bengmark S, et al. Effect of synbiotic therapy on the incidence of ventilator associated pneumonia in critically ill patients: a randomised, double-blind, placebo-controlled trial. Intensive Care Med. 2009; 35:854–61.

46). Kotzampassi K, Giamarellos-Bourboulis EJ, Voudouris A, Kazamias P, Eleftheriadis E. Benefits of a synbiotic formula (Synbiotic 2000Forte) in critically Ill trauma patients: early results of a randomized controlled trial. World J Surg. 2006; 30:1848–55.

47). Spindler-Vesel A, Bengmark S, Vovk I, Cerovic O, Kompan L. Synbiotics, prebiotics, glutamine, or peptide in early enteral nutrition: a randomized study in trauma patients. JPEN J Parenter Enteral Nutr. 2007; 31:119–26.

48). Barraud D, Blard C, Hein F, Marcon O, Cravoisy A, Nace L, et al. Probiotics in the critically ill patient: a double blind, randomized, placebo-controlled trial. Intensive Care Med. 2010; 36:1540–7.

49). Wheeler JG, Shema SJ, Bogle ML, Shirrell MA, Burks AW, Pittler A, et al. Immune and clinical impact of Lactobacillus acidophilus on asthma. Ann Allergy Asthma Immunol. 1997; 79:229–33.
50). Helin T, Haahtela S, Haahtela T. No effect of oral treatment with an intestinal bacterial strain, Lactobacillus rhamnosus (ATCC53103), on birch-pollen allergy: a placebo-controlled double-blind study. Allergy. 2002; 57:243–6.
51). Giovannini M, Agostoni C, Riva E, Salvini F, Ruscitto A, Zuccotti GV, et al. A randomized prospective double blind controlled trial on effects of long-term consumption of fermented milk containing Lactobacillus casei in pre-school children with allergic asthma and/or rhinitis. Pediatr Res. 2007; 62:215–20.
52). Stockert K, Schneider B, Porenta G, Rath R, Nissel H, Eichler I. Laser acupuncture and probiotics in school age children with asthma: a randomized, placebo-controlled pilot study of therapy guided by principles of Traditional Chinese Medicine. Pediatr Allergy Immunol. 2007; 18:160–6.

53). Hamelmann E, Wahn U. Immune responses to allergens early in life: when and why do allergies arise? Clin Exp Allergy. 2002; 32:1679–81.

54). Harata G, He F, Hiruta N, Kawase M, Kubota A, Hiramatsu M, et al. Intranasal administration of Lactobacillus rhamnosus GG protects mice from H1N1 influenza virus infection by regulating respiratory immune responses. Lett Appl Microbiol. 2010; 50:597–602.
55). Racedo S, Villena J, Medina M, Agüero G, Rodríguez V, Alvarez S. Lactobacillus casei administration reduces lung injuries in a Streptococcus pneumoniae infection in mice. Microbes Infect. 2006; 8:2359–66.
56). Fuller R, editor. Probiotics, The Scientific Basis. London: Chapman & Hall;1992.
58). Guglielmetti S, Taverniti V, Minuzzo M, Arioli S, Zanoni I, Stuknyte M, et al. A dairy bacterium displays in vitro probiotic properties for the pharyngeal mucosa by antagonizing group A streptococci and modulating the immune response. Infect Immun. 2010; 78:4734–43.
59). Husni RN, Gordon SM, Washington JA, Longworth DL. Lactobacillus bacteremia and endocarditis: review of 45 cases. Clin Infect Dis. 1997; 25:1048–55.
60). Cannon JP, Lee TA, Bolanos JT, Danziger LH. Pathogenic relevance of Lactobacillus: a retrospective review of over 200 cases. Eur J Clin Microbiol Infect Dis. 2005; 24:31–40.
61). Tannock GW. Conjugal transfer of plasmid pAM beta 1 in Lactobacillus reuteri and between lactobacilli and Enterococcus faecalis. Appl Environ Microbiol. 1987; 53:2693–5.
62). Haug MC, Tanner SA, Lacroix C, Stevens MJ, Meile L. Monitoring horizontal antibiotic resistance gene transfer in a colonic fermentation model. FEMS Microbiol Ecol. 2011; 78:210–9.

63). Klein G, Hallmann C, Casas IA, Abad J, Louwers D, Reuter G. Exclusion of van A, van B and van C type glycopeptide resistance in strains of Lactobacillus reuteri and Lactobacillus rhamnosus used as probiotics by polymerase chain reaction and hybridization methods. J Appl Microbiol. 2000; 89:815–24.
64). Malcata FX, Granja Tavres TS. Hermandez-Mendoza A, Safety of Food and Beverages: Probiotics and Prebiotics Enyclopedia of Food Safety. 2014; 72:129–37.
65). Saarela M, Mogensen G, Fondén R. Mättö J, Mattila-Sandholm T. Probiotic bacteria: safety, functional and technological properties. J Biotechnol. 2000; 84:197–215.
66). Salminen S, Laine M, von Wright A, Vuopio-Varkila J, Korhonen T, Mattila-Sandholm T. Development of selection criteria for probiotic strains to assess their potential in functional foods: a Nordic and European approach. Biosci Microflora. 1996; 15:61–7.

67). Pochart P, Marteau P, Bouhnik Y, Goderel I, Bourlioux P, Rambaud JC. Survival of Bifidobacteria ingested via fermented milk during their passage through the human small intestine: an in vivo study using intestinal perfusion. Am J Clin Nutr. 1992; 55:78–80.
68). Marteau P, Gerhardt MF, Myara A, Bouvier E, Trivin F, Rambaud JC. Metabolism of bile salts by alimentary bacteria during transit in the human small intestine. Microb Ecol Health Dis. 1995; 8:151–7.

69). Korpela R, Moilanen E, Saxelin M, Vapaatalo H. Lactobacillus rhamnosus GG (ATCC 53103) and platelet aggregation in vitro. Int J Food Microbiol. 1997; 37:83–6.
70). Charteris WP, Kelly PM, Morelli L, Collins JK. Antibiotic susceptibility of potentially probiotic Lactobacillus species. J Food Prot. 1998; 61:1636–43.
Figure 1.
Airway composition of microbiota in health and chronic disease. (A) Normal constituents of microbiota, (B) Comparative increase of pathogenic microbial numbers.
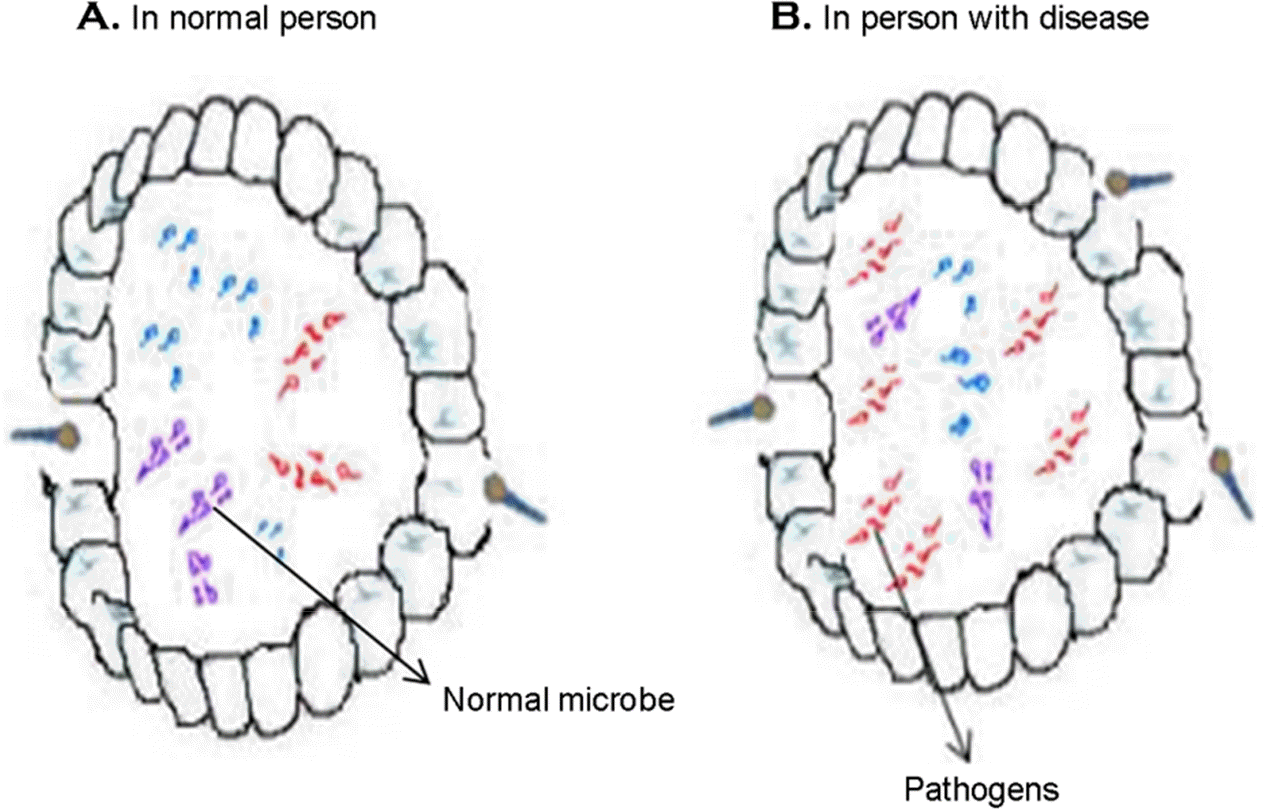
Figure 2.
Types of probiotic activity on epithelial layer. (A) Direct attack, production of antimicrobial factors by probiotics, (B) Inhibition of virulence factors produced by pathogens, (C) Competition for barrier layer between probiotics and pathogens.
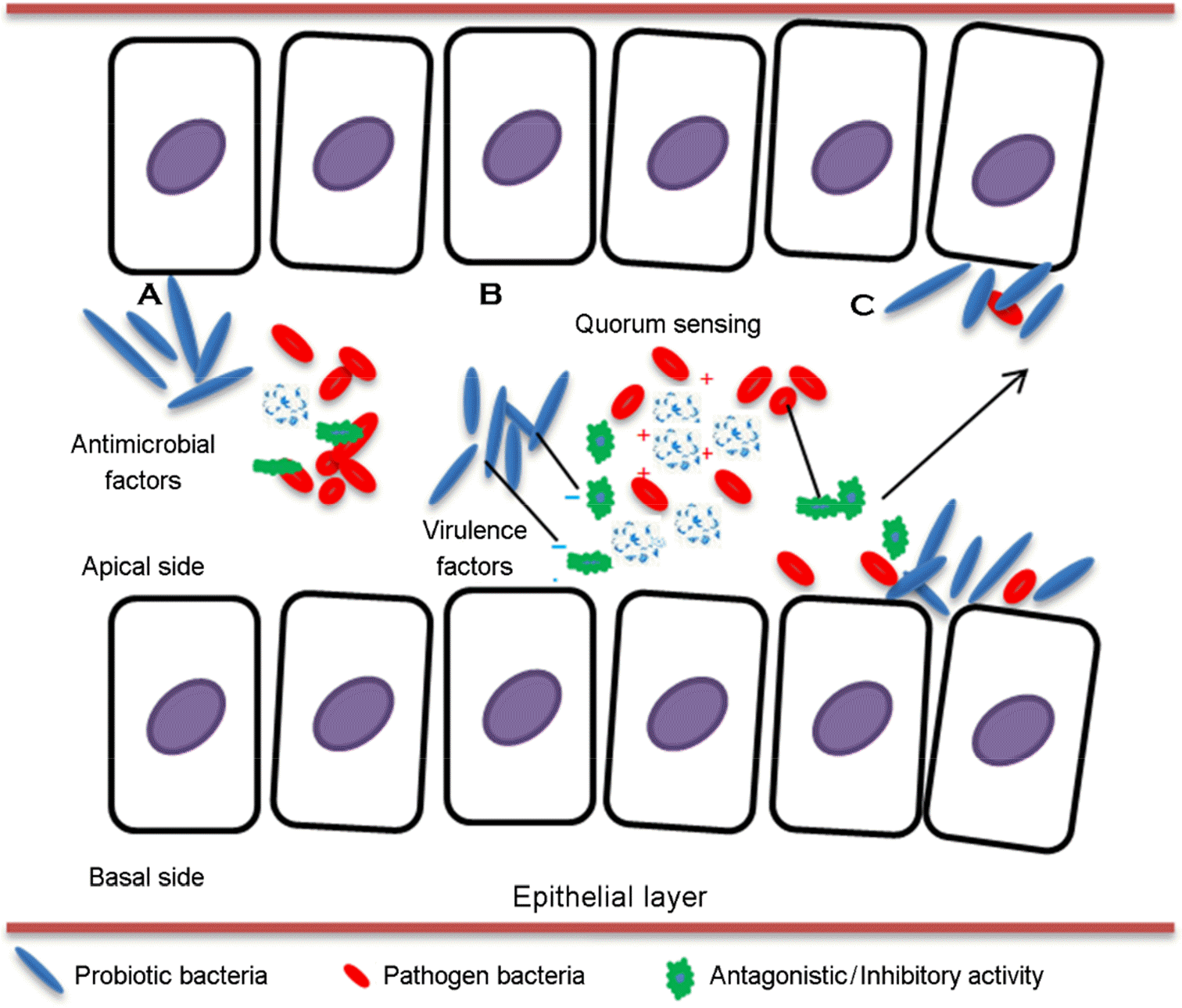
Table 1.
Summaries of double blind randomized, controlled clinical trials for patients of intensive care units after administration of various probiotics on mechanical ventilation pneumonia and mortality
| Reference number | Number of patients Assessed. Probiotics vs. Control | Incidence VAPa, Probiotics vs. Control | Mortality Probiotics vs. Control | Indicator disease |
|---|---|---|---|---|
| 45 | 130 vs. 129 | 9% vs. 13% | 21.5% vs. 26.3% | Pneumonia |
| 43 | 102 vs. 106 | 23% vs. 22% | NAb | Pneumonia |
| 44 | 23 vs. 21 | 4% vs. 14% | 22% vs. 19% | Pneumonia |
| 47 | 26 vs. 87 | 15% vs. 39% | 8% vs. 6% | Pneumonia |
| 46 | 35 vs. 30 | 54% vs. 80% | 14% vs. 30% | Pneumonia |
| 42 | 68 vs. 70 | 19% vs. 40% | 21% vs. 17% | Pneumonia |
| 48 | 87 vs. 80 | 18.7% vs. 26.4% | 25.3% vs. 33.7% | Pneumonia |
Table 2.
Antimicrobial substances produced by probiotic bacteria (56)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download