Abstract
Antiviral activity against Influenza virus of 14 Lactobacillus species isolated from food was monitored. Lactobacillus species were isolated from traditional Korean fermented food. Each live Lactobacillus was administered into the nasal cavity of SPF 6-week-old BALB/c mice. After the Lactobacillus treatment, Influenza virus (A/NWS/33/H1N1) was inoculated to each mouse. Clinical signs and mortality was monitored for 21 days. Each Lactobacillus strain showed various level of antiviral activity against Influenza virus. As a result of this study, this mouse experiment model, including intranasal treatment of live Lactobacillus species, could be effective model in evaluating immunomodulatory response of probiotics against respiratory viruses.
REFERENCES
1). Widmer K1, Zhu Y, Williams JV, Griffin MR, Edwards KM, Talbot HK. Rates of hospitalizations for respiratory syncytial virus, human metapneumovirus, and influenza virus in older adults. J Infect Dis. 2012; 206:56–62.

2). König R, Stertz S, Zhou Y, Inoue A, Hoffmann HH, Bhattacharyya S, et al. Human host factors required for influenza virus replication. Nature. 2010; 463:813–7.

3). Mesch GS, Schwirian KP, Kolobov T. Attention to the media and worry over becoming infected: the case of the Swine Flu (H1N1) Epidemic of 2009. Sociol Health Illn. 2013; 35:325–31.

4). Puvanalingam A, Rajendiran C, Sivasubramanian K, Ragunanthanan S, Suresh S, Gopalakrishnan S. Case series study of the clinical profile of H1N1 swine flu influenza. J Assoc Physicians India. 2011; 59:14–6.
5). Chiu SS, Lo JY, Chan KH, Chan EL, So LY, Wu P, et al. Population-based hospitalization burden of influenza a virus subtypes and antigenic drift variants in children in Hong Kong (2004–2011). PLoS One. 2014; 9:e92914.

6). Li GM, Chiu C, Wrammert J, McCausland M, Andrews SF, Zheng NY, et al. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc Natl Acad Sci U S A. 2012; 109:9047–52.

7). Ramos I, Bernal-Rubio D, Durham N, Belicha-Villanueva A, Lowen AC, Steel J, et al. Effects of receptor binding specificity of avian influenza virus on the human innate immune response. J Virol. 2011; 85:4421–31.

8). Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011; 470:543–7.

9). Guarner FL, Khan AG, Garisch J, Eliakim R, Gangl A, Thomson A, et al. World Gastroenterology Organisation Global Guidelines: probiotics and prebiotics October 2011. J Clin Gastroenterol. 2012; 46:468–81.
10). Weichert S, Schroten H, Adam R. The role of prebiotics and probiotics in prevention and treatment of childhood infectious diseases. Pediatr Infect Dis J. 2012; 31:859–62.

11). Aboubakr HA, El-Banna AA, Youssef MM, Al-Sohaimy SA, Goyal SM. Antiviral Effects of Lactococcus lactis on Feline Calicivirus, A Human Norovirus Surrogate. Food Environ Virol. 2014.

12). Kaboosi H. Antibacterial effects of probiotics isolated from yoghurts against some common bacterial pathogens. Afr J Microbiol Res. 2011; 5:4363–7.

13). Oh MH, Lee SG, Paik SY. Antiviral Activity of Lactobacillus spp. and Polysaccharide. J Bacteriol Virol. 2010; 40:145–50.
14). Albesharat R, Ehrmann MA, Korakli M, Yazaji S, Vogel RF. Phenotypic and genotypic analyses of lactic acid bacteria in local fermented food, breast milk and faeces of mothers and their babies. Syst Appl Microbiol. 2011; 34:148–55.

15). Zhang Y, Zhang L, Du M, Yi H, Guo C, Tuo Y, et al. Antimicrobial activity against Shigella sonnei and probiotic properties of wild lactobacilli from fermented food. Microbiol Res. 2011; 167:27–31.
16). Youn HN, Lee DH, Lee YN, Park JK, Yuk SS, Yang SY, et al. Intranasal administration of live Lactobacillus species facilitates protection against influenza virus infection in mice. Antiviral Res. 2012; 93:138–43.
17). Franz CM, Huch M, Mathara JM, Abriouel H, Benomar N, Reid G, et al. African fermented foods and probiotics. Int J Food Microbiol. 2014; 190:84–96.

18). Champagne CP, Ross RP, Saarela M, Hansen KF, Charalampopoulos D. Recommendations for the viability assessment of probiotics as concentrated cultures and in food matrices. Int J Food Microbiol. 2011; 149:185–93.

19). Jang SE, Hyun YJ, Oh YJ, Choi KB, Kim T, Yeo IH, et al. Adhesion Activity of Lactobacillus plantarum PM 008 Isolated from Kimchi on the Intestine of Mice. J Bacteriol Virol. 2011; 41:83–90.
20). Harata G, He F, Hiruta N, Kawase M, Kubota A, Hiramatsu M, et al. Intranasal administration of Lactobacillus rhamnosus GG protects mice from H1N1 influenza virus infection by regulating respiratory immune responses. Lett Appl Microbiol. 2010; 50:597–602.
22). Rizzello V, Bonaccorsi I, Dongarrà ML, Fink LN, Ferlazzo G. Role of Natural Killer and Dendritic Cell Crosstalk in Immunomodulation by Commensal Bacteria Probiotics. J Biomed Biotechnol. 2011.

23). Yang OO, Kelesidis T, Cordova R, Khanlou H. Immunomodulation of Antiretroviral Drug-Suppressed Chronic HIV-1 Infection in an Oral Probiotic Double-Blind Placebo-Controlled Trial. AIDS Res Hum Retroviruses. 2014; 30:988–95.

24). Lim YJ, Jo YH, Kim HJ, Park JK. The Synergistic Effects of Antimicrobial Peptides on the Growth Inhibition of Salmonella Typhimurium through Imd Pathway in Drosophila Intestine. J Bacteriol Virol. 2013; 43:120–30.
25). Hori T, Kiyoshima J, Shida K, Yasui H. Effect of intranasal administration of Lactobacillus casei Shirota on influenza virus infection of upper respiratory tract in mice. Clin Diagn Lab Immunol. 2011; 8:593–7.
26). Jin D, Kim BW, Cho HC, Yoon SS, Park YS, Kim SK. Immunoregulation of Murine Immunocytes to Bifidobacteria Strain Isolated from Feces of Healthy Korean Children: IL-10 Release and Proportional Change of CD4+CD25+ Cells. J Bacteriol Virol. 2010; 40:171–7.
Figure 1.
Schedule for animal experiment. BALB/c mice were treated with intranasally with Lactobacillus for 2 weeks before inoculation with A/NWS/33 virus. Mice were monitored daily for clinical signs for 14 days.
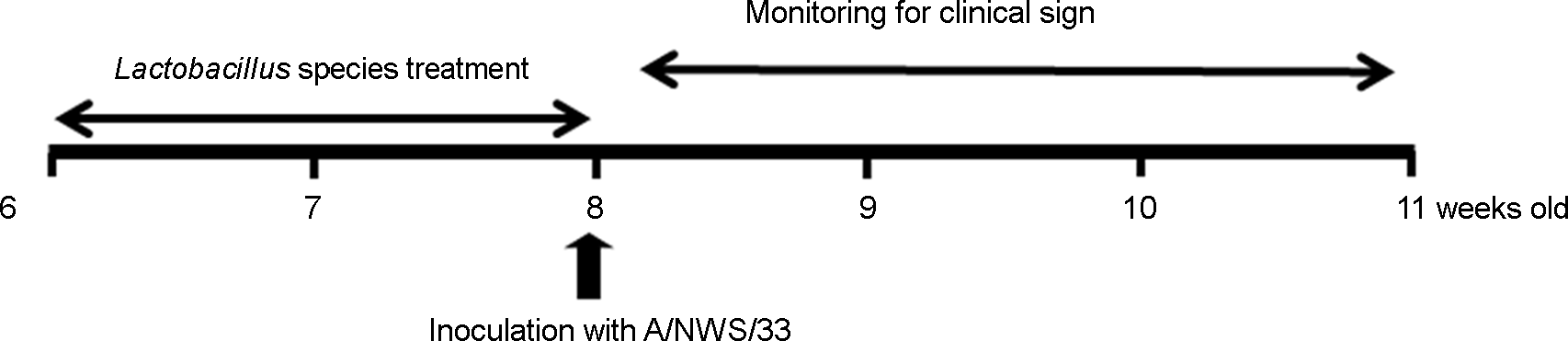
Figure 2.
Rooted neighbor-joining tree based on 16s rRNA sequences showing relationships between Lactobacillus strains. The scale bar indicates 0.1 nucleotide substitution per nucleotide position.
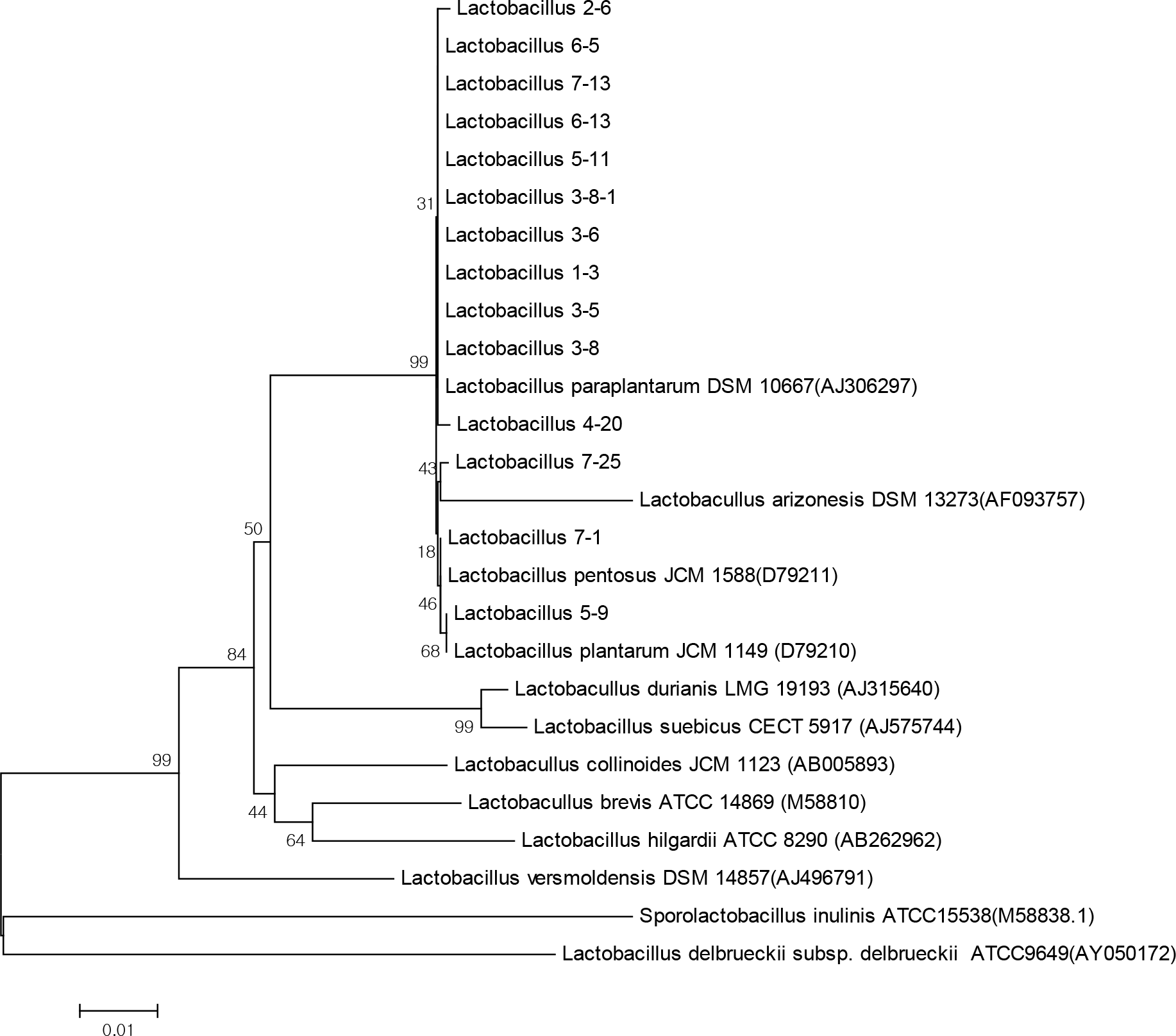
Table 1.
Origin of isolated Lactobacillus strains
| Lactobacillus strain No. | Origin | Genetic ClusterA |
|---|---|---|
| Lactobacillus strain No. 1–3 | Pickled Radish | Group 1 |
| Lactobacillus strain No. 2–6 | Pickled Pepper | Group 1 |
| Lactobacillus strain No. 3–5 | Pickled Pepper | Group 1 |
| Lactobacillus strain No. 3–6 | Dongchimi | Group 1 |
| Lactobacillus strain No. 3–8 | Pickled Pepper | Group 1 |
| Lactobacillus strain No. 3–8-1 | Dongchimi | Group 1 |
| Lactobacillus strain No. 4–20 | Pickled Radish | Group 1 |
| Lactobacillus strain No. 5–9 | Dongchimi | Group 4 |
| Lactobacillus strain No. 5–11 | Salted shrimp | Group 1 |
| Lactobacillus strain No. 6–5 | Pickled Pepper | Group 1 |
| Lactobacillus strain No. 6–13 | White Kimchi | Group 1 |
| Lactobacillus strain No. 7–1 | Dongchimi | Group 3 |
| Lactobacillus strain No. 7–13 | Pickled Pepper | |
| Lactobacillus strain No. 7–25 | Salted shrimp | Group 2 |
Table 2.
The effect of intranasal administration of various Lactobacillus species on challenge with influenza virus. BALB/c mice were treated intranasally with Lactobacillus species for 2 weeks before inoculation with A/NWS/33 virus. Mice were monitored daily for clinical signs for 21 days. Statistical significance was determined by Fisher's exact test. Asterisks indicates significant differences (*p < 0.05, **p < 0.01) compared with results in positive control
| Group | Administered Lactobacillus species | Number of mice |
Preventive effect after challenge with influenza virusA |
PI14C | |
|---|---|---|---|---|---|
| Survival | MDT (day)B | ||||
| 1 | Lactobacillus 1–3 | 10 | 6/10 | 10.0 | 1.4 |
| 2 | Lactobacillus 2–6 | 10 | 8/10** | 9.0 | 1.2 |
| 3 | Lactobacillus 3–5 | 10 | 8/10** | 10.5 | 1.2 |
| 4 | Lactobacillus 3–6 | 10 | 4/10 | 10.5 | 1.6 |
| 5 | Lactobacillus 3–8 | 10 | 8/10** | 12.0 | 1.2 |
| 6 | Lactobacillus 3-8-1 | 10 | 4/10 | 8.5 | 1.6 |
| 7 | Lactobacillus 4–20 | 10 | 3/10 | 10.1 | 1.7 |
| 8 | Lactobacillus 5–9 | 10 | 4/10 | 10.8 | 1.6 |
| 9 | Lactobacillus 5–11 | 10 | 5/10 | 8.4 | 1.5 |
| 10 | Lactobacillus 6–5 | 10 | 8/10** | 11.0 | 1.2 |
| 11 | Lactobacillus 6–13 | 10 | 5/10 | 10.6 | 1.5 |
| 12 | Lactobacillus 7-1 | 10 | 2/10 | 10.5 | 1.8 |
| 13 | Lactobacillus 7-13 | 10 | 5/10 | 9.0 | 1.5 |
| 14 | Lactobacillus 7–25 | 10 | 6/10 | 9.8 | 1.4 |
| 15 | Positive control | 10 | 1/10 | 9.0 | 1.9 |
| 16 | Negative control | 10 | 10/10 | − | 0 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download