Abstract
Hepatitis C virus (HCV) is one of the main causes of liver disease. 1~2% of the Korean people has been reported to be infected by HCV. Although HCV is less infectious than hepatitis B virus (HBV), it is more prone to develop chronic infection (~ 80%) which may link to cirrhosis and hepatocellular carcinogenesis. In addition, prevalence of hepatitis caused by HCV infection is gradually increased every year in Korea. Recently, a large number of clinical trials using direct-acting antiviral (DAA) drugs have been shown efficient therapeutic results for chronic HCV infections and some of them are on the market. However, there is still a concern on viral evasion to the DAAs and the effective mechanisms of immunological clearance of HCV remains to be elucidated. Here, we introduce the recent findings on the role of Th17-Treg axis which may play a critical role of the viral pathogenesis and/or immunological defense against HCV infection. The underlying regulatory mechanisms of Th17-Treg axis might be a potential candidate for the better control of HCV chronic infections.
Go to : 
REFERENCES
1). Park SY, Rim MY, Yo IK, Ha MS, Kim JS, Lee JW, et al. [Efficacy of peginterferon and ribavirin combination therapy of chronic hepatitis C: a pooled analysis]. Korean J Gastroenterol. 2012; 60:306–14.

2). Heim MH. 25 years of interferon-based treatment of chronic hepatitis C: an epoch coming to an end. Nat Rev Immunol. 2013; 13:535–42.

3). Muir AJ, Naggie S. Hepatitis C Virus Treatment: Is It Possible To Cure All Hepatitis C Virus Patients? Clin Gastroenterol Hepatol. 2015; 13:2166–72.
4). Chung RT, Baumert TF. Curing chronic hepatitis C–the arc of a medical triumph. N Engl J Med. 2014; 370:1576–8.
5). Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001; 358:958–65.

6). Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL Jr, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002; 347:975–82.

7). Coppola N, Pisaturo M, Zampino R, Macera M, Sagnelli C, Sagnelli E. Hepatitis C virus markers in infection by hepatitis C virus: In the era of directly acting antivirals. World J Gastroenterol. 2015; 21:10749–59.

8). Cotte L, Barrail-Tran A, Vincent C, Valantin MA, Fournier I, Lacombe K, et al. Telaprevir enhances ribavirin-induced anaemia through renal function impairment. Antivir Ther. 2015; 20:479–86.

9). Heim MH, Thimme R. Innate and adaptive immune responses in HCV infections. J Hepatol. 2014; 61:S14–25.

10). Lemmers A, Moreno C, Gustot T, Maréchal R, Degré D, Demetter P, et al. The interleukin-17 pathway is involved in human alcoholic liver disease. Hepatology. 2009; 49:646–57.

11). Chang Q, Wang YK, Zhao Q, Wang CZ, Hu YZ, Wu BY. Th17 cells are increased with severity of liver inflammation in patients with chronic hepatitis C. J Gastroenterol Hepatol. 2012; 27:273–8.

12). Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007; 25:821–52.

13). Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012; 11:763–76.

14). Lockhart E, Green AM, Flynn JL. IL-17 production is ominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006; 177:4662–9.
15). Cupedo T, Crellin NK, Papazian N, Rombouts EJ, Weijer K, Grogan JL, et al. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol. 2009; 10:66–74.

16). Pandiyan P, Zhu J. Origin and functions of pro-inflammatory cytokine producing Foxp3 (+) regulatory T cells. Cytokine. 2015; 76:13–24.
17). Hao C, Zhou Y, He Y, Fan C, Sun L, Wei X, et al. Imbalance of regulatory T cells and T helper type 17 cells in patients with chronic hepatitis C. Immunology. 2014; 143:531–8.

18). Wang JP, Zhang Y, Wei X, Li J, Nan XP, Yu HT, et al. Circulating Toll-like receptor (TLR) 2, TLR4, and regulatory T cells in patients with chronic hepatitis C. APMIS. 2010; 118:261–70.

19). Cabrera R, Tu Z, Xu Y, Firpi RJ, Rosen HR, Liu C, et al. An immunomodulatory role for CD4 (+)CD25 (+) regulatory T lymphocytes in hepatitis C virus infection. Hepatology. 2004; 40:1062–71.
20). Yu JW, Wang GQ, Sun LJ, Li XG, Li SC. Predictive value of rapid virological response and early virological response on sustained virological response in HCV patients treated with pegylated interferon alpha-2a and ribavirin. J Gastroenterol Hepatol. 2007; 22:832–6.
Go to : 
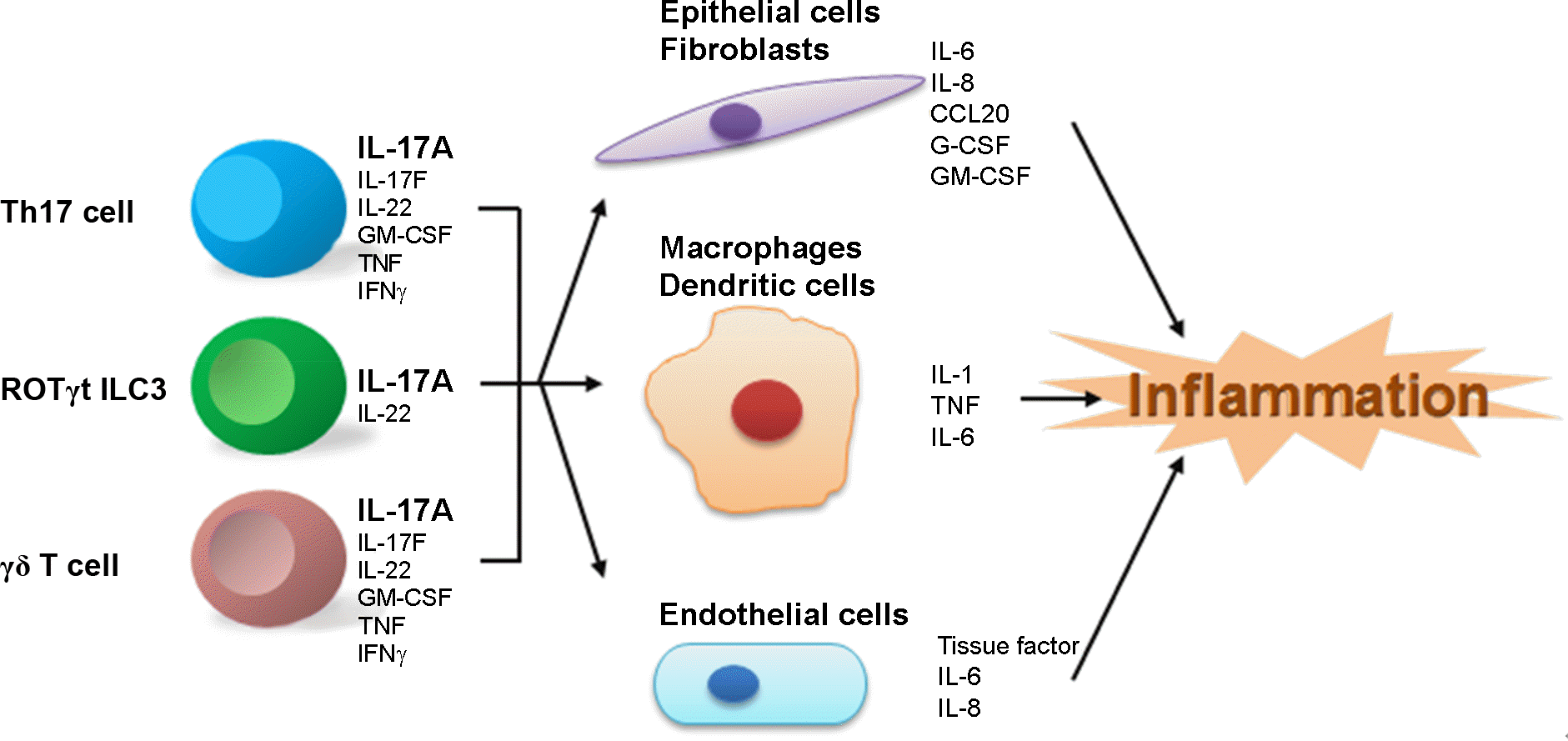 | Figure 1.Subsets of immune cells producing IL-17 and their targets. There are three major subsets of IL-17-producing cells; T helper 17(Th17), RORγt+ innate lymphoid cells (IL-17+ ILC3) and gamma-delta T cells (γδ T cells). IL-17 produced from these cells contributes to inflammation by increasing production of inflammatory cytokines from the target cells, such as epithelial cells, fibroblasts, macrophages, dendritic cells, and endothelial cells. CCL20, chemokine CC motif ligand 20; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage CSF. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download