Abstract
Vibrio vulnificus causes fatal infections in susceptible individuals. Group 1 capsular polysaccharide (CPS) operon is responsible for CPS expression, which plays an essential role in the pathogenesis of this pathogen. Cyclic AMP (cAMP) and cAMP receptor protein (Crp) complex, which responds to glucose availability and functions as a global regulator, has been known to affect CPS production in this pathogen. This study was undertaken to experimentally verify whether cAMP-Crp directly or indirectly affects CPS production. A mutation in cyaA encoding adenylate cyclase, which is required for cAMP biosynthesis, inhibited V. vulnificus growth and changed opaque colonies to translucent colonies, and these changes were recovered by complementing cyaA or by adding exogenous cAMP. A mutation in crp encoding Crp also inhibited V. vulnificus growth and changed opaque colonies to translucent colonies, and these changes were recovered by complementing crp. Moreover, the crp or cyaA mutation decreased the susceptibility of V. vulnificus against NaOCl. The crp mutation reduced the transcription levels of group 1 CPS operon on a per cell basis. Glucose addition in the absence of Crp stimulated V. vulnificus growth, changed translucent colonies to opaque colonies, and increased the transcription levels of group 1 CPS operon. These results indicate that cAMP or Crp is indirectly involved in optimal CPS production by positively affecting metabolism or V. vulnificus growth rather than by directly controlling the expression of group 1 CPS operon.
Go to : 
REFERENCES
1). Jones MK, Oliver JD. Vibrio vulnificus: disease and pathogenesis. Infect Immun. 2009; 77:1723–33.
2). Kim YR, Kim SY, Kim CM, Lee SE, Rhee JH. Essential role of an adenylate cyclase in regulating Vibrio vulnificus virulence. FEMS Microbiol Lett. 2005; 243:497–503.
3). Choi MH, Sun HY, Park RY, Kim CM, Bai YH, Kim YR, et al. Effect of the crp mutation on the utilization of transferrin-bound iron by Vibrio vulnificus. FEMS Microbiol Lett. 2006; 257:285–92.
4). Kim YR, Lee SE, Kim B, Choy H, Rhee JH. A dual regulatory role of cyclic adenosine monophosphate receptor protein in various virulence traits of Vibrio vulnificus. Microbiol Immunol. 2013; 57:273–80.
5). Lee JH, Rhee JE, Park U, Ju HM, Lee BC, Kim TS, et al. Identification and functional analysis of Vibrio vulnificus SmcR, a novel global regulator. J Microbiol Biotech. 2007; 17:325–34.
6). Hülsmann A, Rosche TM, Kong IS, Hassan HM, Beam DM, Oliver JD. RpoS-dependent stress response and exoenzyme production in Vibrio vulnificus. Appl Environ Microbiol. 2003; 69:6114–20.
7). Yoshida S, Ogawa M, Mizuguchi Y. Relation of capsular materials and colony opacity to virulence Vibrio vulnificus. Infect Immun. 1985; 47:446–51.
8). Simpson LM, White VK, Zane SF, Oliver JD. Correlation between virulence and colony morphology in Vibrio vulnificus. Infect Immun. 1987; 55:269–72.
9). Wright AC, Simpson LM, Oliver JD, Morris JG Jr. Phenotypic evaluation of acapsular transposon mutants of Vibrio vulnificus. Infect Immun. 1990; 58:1769–73.
10). Shinoda S, Kobayashi M, Yamada H, Yoshida S, Ogawa M, et al. Inhibitory effect of capsular antigen of Vibrio vulnificus on bactericidal activity of human serum. Microbiol Immunol. 1989; 31:393–401.
11). Powell JL, Wright AC, Wasserman SS, Hone DM, Morris JG Jr. Release of tumor necrosis factor alpha in response to Vibrio vulnificus capsular polysaccharide in in vivo and in vitro models. Infect Immun. 1997; 65:3713–8.
12). Tamplin ML, Specter S, Rodrick GE, Friedman H. Vibrio vulnificus resists phagocytosis in the absence of serum opsonins. Infect Immun. 1985; 49:715–8.
13). Kreger AS, Gray LD, Testa J. Protection of mice against Vibrio vulnificus disease by vaccination with surface antigen preparation and anti-surface antigen antisera. Infect Immun. 1984; 45:537–43.
14). Devi SJ, Hayat U, Powell JL, Morris JG Jr. Preclinical immunoprophylactic and immunotherapeutic efficacy of antisera to capsular polysaccharide-tetanus toxoid conjugate vaccines of Vibrio vulnificus. Infect Immun. 1996; 64:2220–4.
15). Kim YR, Rhee JH. Flagellar basal body flg operon as a virulence determinant of Vibrio vulnificus. Biochem Biophys Res Commun. 2003; 304:405–10.
16). Paranjpye RN, Lara JC, Pepe JC, Pepe CM, Strom MS. The type IV leader peptidase/N-methyltransferase of Vibrio vulnificus controls factors required for adherence to Hep-2 cells and virulence in iron-overloaded mice. Infect Immun. 1998; 66:5659–68.
17). Starks AM, Schoeb TR, Tamplin ML, Parveen S, Doyle TJ, Bomeisl PE, et al. Pathogenesis of infection by clinical and environmental strains of Vibrio vulnificus in iron dextran-treated mice. Infect Immun. 2000; 68:5785–93.
18). Kim YR, Lee SE, Kook H, Yeom JA, Na HS, Kim SY, et al. Vibrio vulnificus RTX toxin kills host cells only after contact of the bacteria with host cells. Cell Microbiol. 2008; 10:848–62.
19). Zuppardo AB, Siebeling RJ. An epimerase gene essential for capsule synthesis in Vibrio vulnificus. Infect Immun. 1998; 66:2601–6.
20). Wright AC, Powell JL, Kaper JB, Morris JG Jr. Identification of a group 1-like capsular polysaccharide operon for Vibrio vulnificus. Infect Immun. 2001; 69:6893–901.
21). Smith AB, Siebeling RJ. Identification of genetic loci required for capsular expression in Vibrio vulnificus. Infect Immun. 2003; 71:1091–7.
22). Reddy GP, Hayat U, Abeygunawardana C, Fox C, Wright AC, Maneval DR Jr, et al. Purification and determination of the structure of capsular polysaccharide of Vibrio vulnificus M06-24. J Bacteriol. 1992; 174:2620–30.
23). Bush CA, Patel P, Gunawardena S, Powell J, Joseph A, Johnson JA, et al. Classification of Vibrio vulnificus strains by the carbohydrate composition of their capsular polysaccharides. Anal Biochem. 1997; 250:186–95.
24). Chatzidaki-Livanis M, Jones MK, Wright AC. Genetic variation in the Vibrio vulnificus group 1 polysaccharide operon. J Bacteriol. 2006; 188:1987–98.
25). Wright AC, Powell JL, Tanner MK, Ensor LA, Karpas AB, Morris JG Jr, et al. Differential expression of Vibrio vulnificus capsular polysaccharide. Infect Immun. 1999; 67:2250–7.
26). Hilton T, Rosche T, Froelich B, Smith B, Oliver J. Capsular polysaccharide phase variation in Vibrio vulnificus. Appl Environ Microbiol. 2006; 72:6986–93.
27). Deutscher J. The mechanisms of carbon catabolite repression in bacteria. Curr Opin Microbiol. 2008; 11:87–93.

28). Bang YB, Lee SE, Rhee JH, Choi SH. Evidence that expression of the Vibrio vulnificus hemolysin gene is dependent on cyclic AMP and cyclic AMP receptor protein. J Bacteriol. 1999; 181:7639–42.
29). Jeong HS, Lee MH, Lee KH, Park SJ, Choi SH. SmcR and cyclic AMP-receptor protein coactivate Vibrio vulnificus vvpE encoding elastase through the RpoS-dependent promoter in a synergistic manner. J Biol Chem. 2003; 278:45072–81.
30). Oh MH, Lee SM, Lee DH, Choi SH. Regulation of the Vibrio vulnificus hupA gene by temperature alteration and cyclic AMP receptor protein and evaluation of its role in virulence. Infect Immun. 2009; 77:1208–15.
31). Grau BL, Henk MC, Garrison KL, Olivier BJ, Schulz RM, O'Reilly KL, et al. Further characterization of Vibrio vulnificus rugose variants and identification of a capsular and rugose exopolysaccharide gene cluster. Infect Immun. 2008; 76:1485–97.
32). Kim SY, Lee SE, Kim YR, Kim CM, Ryu PY, Choy HE, et al. Regulation of Vibrio vulnificus virulence by LuxS quorum-sensing system. Mol Microbiol. 2003; 48:1647–64.
33). McGee K, Hörstedt P, Milton DL. Identification and characterization of additional flagellin genes from Vibrio anguillarum. J Bacteriol. 1996; 178:5188–98.
34). Miller VL, Mekalanos JJ. A novel suicide vector and its use in construction of insertion mutations: Osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988; 170:2575–83.
35). Farinha MA, Kropinski AM. Construction of broad-host-range plasmid vectors for easy visible selection and analysis of promoters. J Bacteriol. 1990; 172:3496–9.

36). Ditta G, Stanfield S, Corbin D, Helinski DR. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980; 77:7347–51.
37). Miller JH. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. New York: Cold Spring Harbor, Cold Spring Harbor Laboratory Press;1992.
38). Kim CM, Shin SH. Regulation of the Vibrio vulnificus vvpE expression by cyclic AMP-receptor protein and quorum-sensing regulator SmcR. Microb Pathog. 2010; 49:348–53.
39). Kim CM, Kim SJ, Shin SH. Cyclic AMP-receptor protein activates aerobactin receptor IutA expression in Vibrio vulnificus. J Microbiol. 2012; 50:320–5.
40). Masson L, Holbein BE. Influence of nutrient limitation and low pH on serogroup B Neisseria meningitidis capsular polysaccharide levels: correlation with virulence for mice. Infect Immun. 1985; 47:465–71.
41). Paoletti LC, Ross RA, Johnson KD. Cell growth rate regulates expression of group B streptococcus type III capsular polysaccharide. Infect Immun. 1996; 64:1220–6.
42). Sellin M, Håkansson S, Norgren M. Phase-shift of polysaccharide capsule expression in group B streptococci, type III. Microb Pathog. 1995; 18:401–15.

43). Vermeulen C, Cross A, Byrne WR, Zollinger W. Quantitative relationship between capsular content and killing of K1-encapsulated Escherichia coli. Infect Immun. 1988; 56:2723–30.
44). Goncalves VM, Zangirolami TC, Giordano RL, Raw I, Tanizaki MM, Giordano RC. Optimization of medium and cultivation conditions for capsular polysaccharide production by Streptococcus pneumoniae serotype 23F. Appl Microbiol Biotechnol. 2002; 59:713–7.
45). Zhang Z, Gosset G, Barabote R, Gonzalez CS, Cuevas WA, Saier MH Jr. Functional interactions between the carbon and iron utilization regulators, Crp and Fur, in Escherichia coli. J Bacteriol. 2005; 187:980–90.
Go to : 
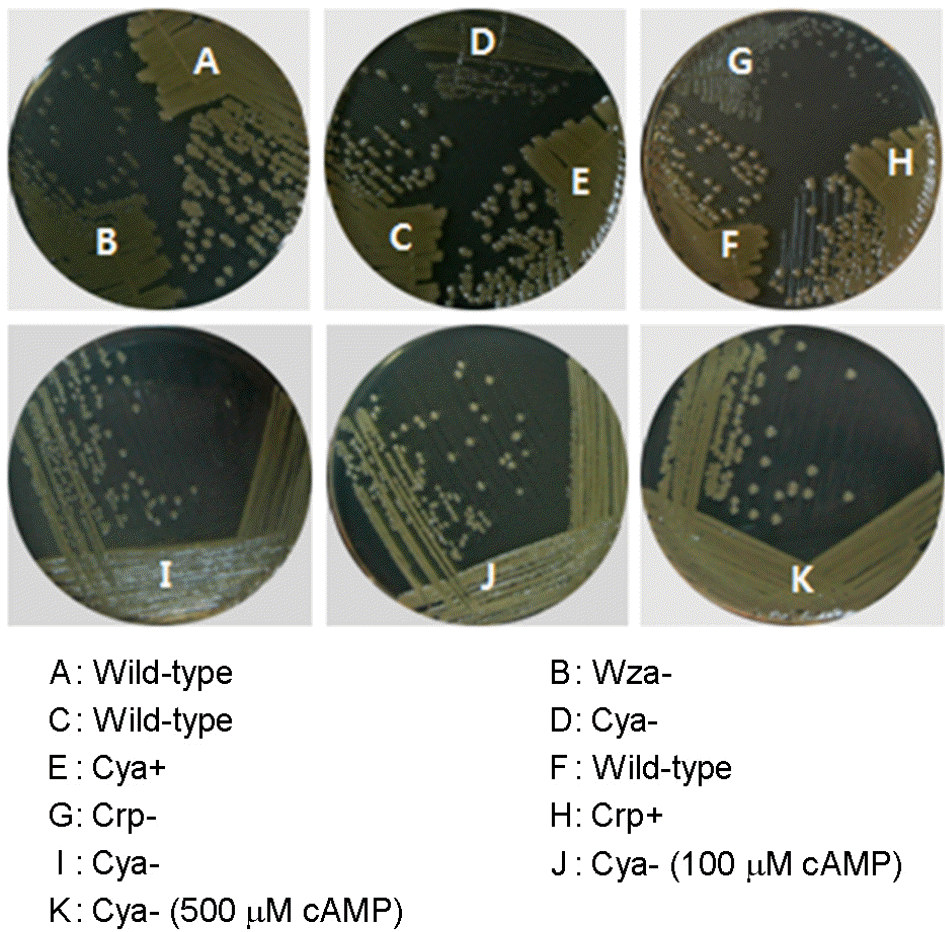 | Figure 1.Colony morphologies of Vibrio vulnificus strains under different conditions. V. vulnificus strains were spread on the surfaces of HI agars or HI agars containing 100 μM cAMP, and cultured for 24 h at 37°C. Wild-type: M06-24/O, Wza-: RC398 containing mutated wza, Cya-: RC386 containing mutated cya, Cya+: RC390 containing complemented cya, Crp-: CMM710 containing mutated crp, Crp+: CMM714 containing complemented crp.
|
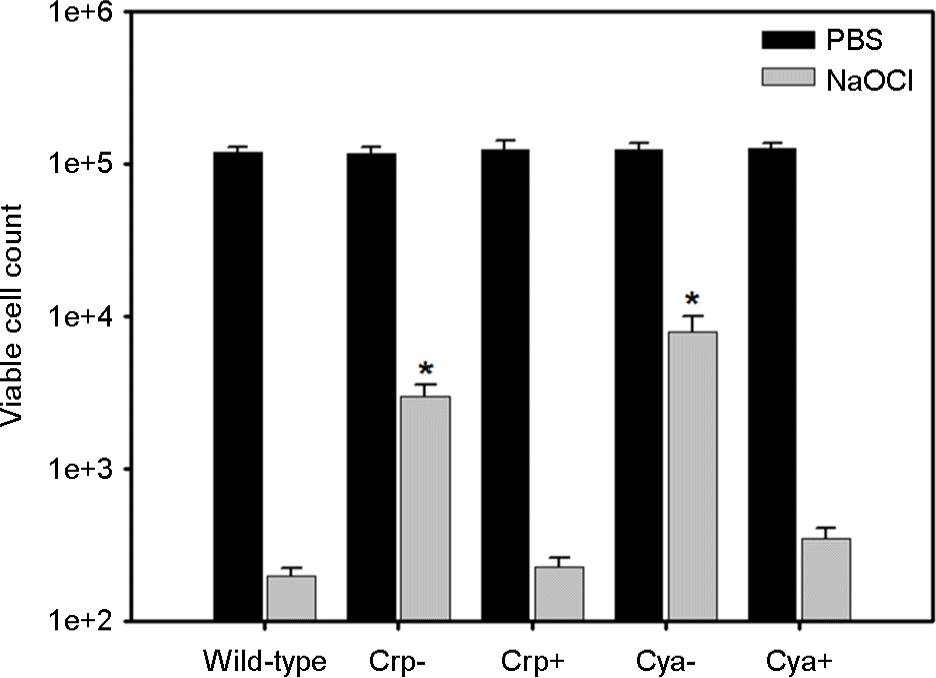 | Figure 2.Effect of cyaA or crp mutation on chlorine resistance. For each V. vulnificus strain, approximately 105 mid-exponentialphase cells were exposed to either phosphate-buffered saline (PBS, pH 7.2) or 1.5 μg/ml NaOCl for 5 min, which was followed by dilution and plating. The experiments were conducted at least three times, and viable cell count was expressed as the log mean (large bar) and standard deviation (error bar) of triplicate measurements (*: p < 0.05 in two-way ANOVA). Wild-type: M06-24/O, Crp-: CMM710 containing mutated crp, Crp+: CMM714 containing complemented crp, Cya-: RC386 containing mutated cya, Cya+: RC390 containing complemented cya.
|
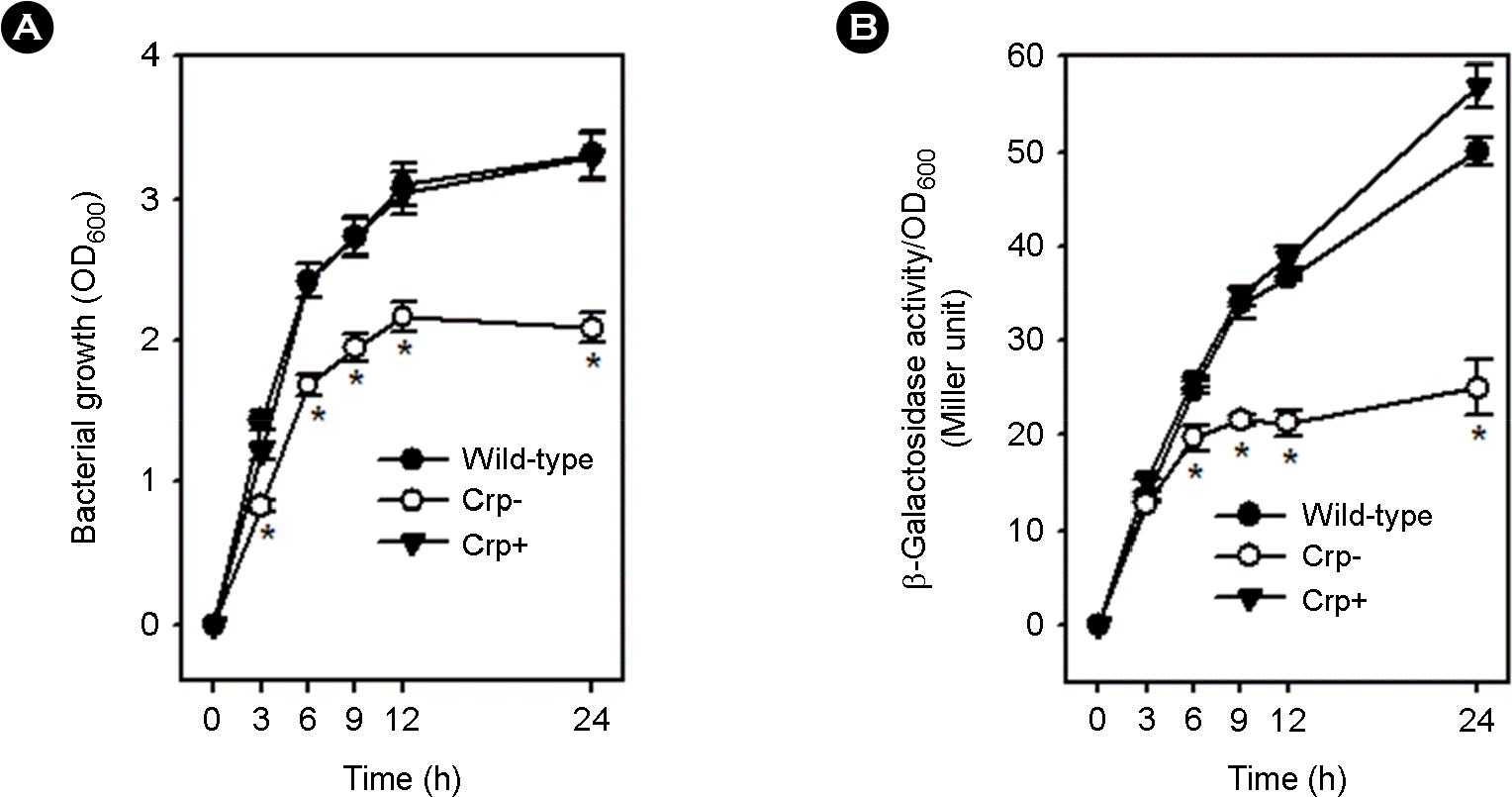 | Figure 3.Effect of Crp on the expression of group 1 CPS operon. The three strains containing the Pwza::lacZ fusion construct, that is, RC342 containing wild-type crp (Wild-type), RC344 containing mutated crp (Crp-) and RC360 containing complemented crp (Crp-), were cultured in HI broths for 24 h at 37°C. Bacterial growths were determined by measuring optical densities of culture aliquots at 600 nm (OD600), and wza transcription was quantified by measuring β-galactosidase activity on a per cell basis (Miller units) in culture aliquots. Bacterial growth levels and β-galactosidase activities are the means and standard deviations (error bars) of triplicate measurements (*: p < 0.05 in Student's t-test). |
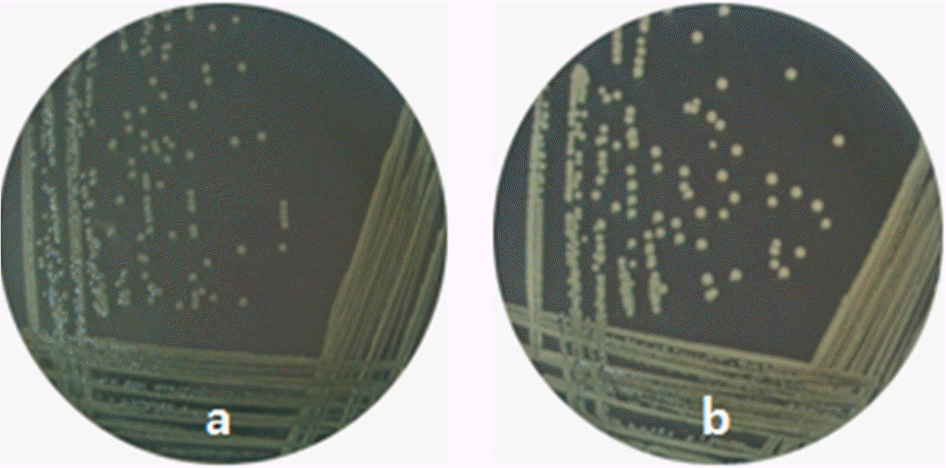 | Figure 4.Effect of glucose on the colony opacity of Vibrio vulnificus in the absence of Crp. CMM714 containing mutated crp was spread on the surface of TES-HI agars containing 0.0 (a) or 0.25% (b) glucose and cultured at 37°C overnight. |
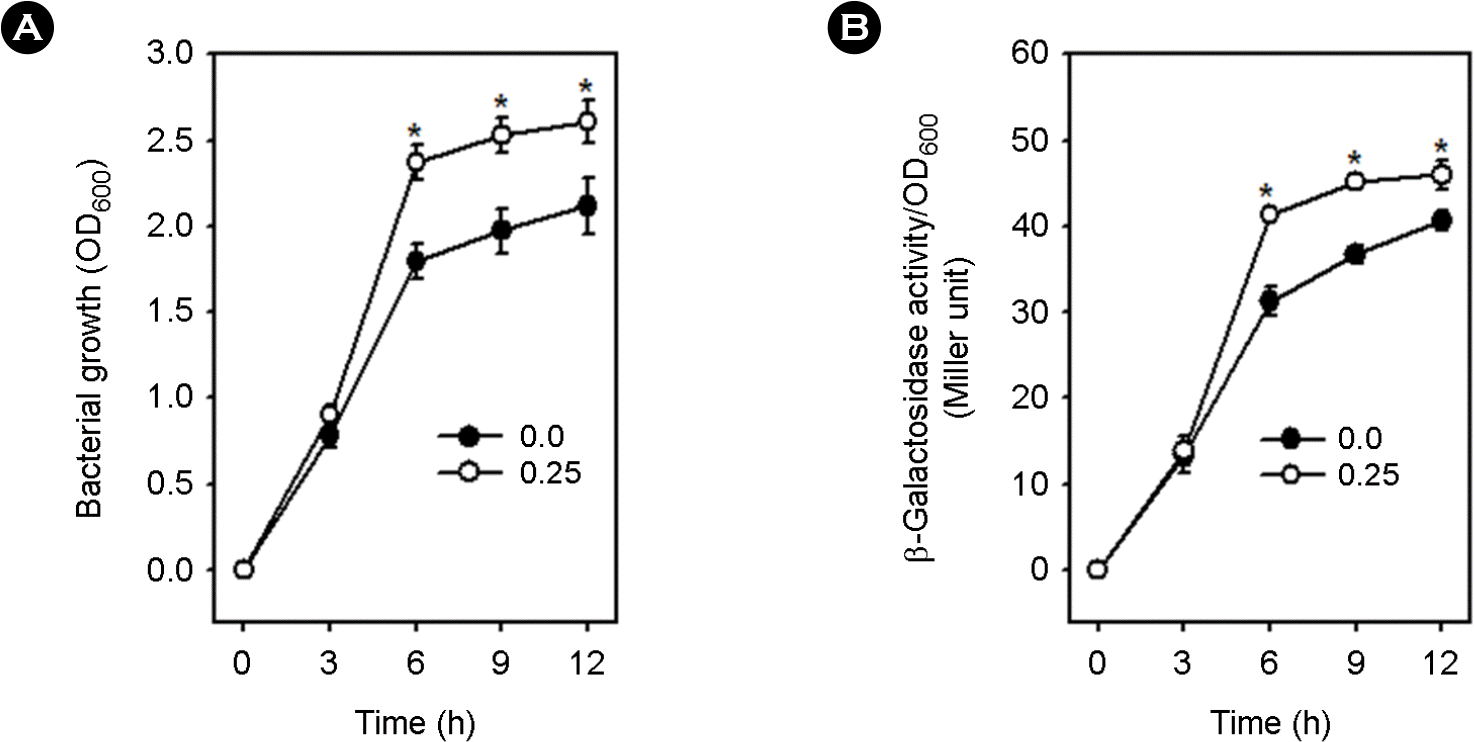 | Figure 5.Effect of glucose on Vibrio vulnificus growth (A) and the expression of group 1 CPS operon (B) in the absence of Crp. RC344 containing mutated crp and the Pwza::lacZ fusion construct was cultured in TES-HI broths containing 0.0 or 0.25% glucose for 24 h at 37°C. Growth and wza transcription were determined and expressed as described in the legend of Fig. 2 (*: p < 0.05 in Student's t-test). |
Table 1.
Bacterial strains, plasmids and primers used in this study




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download