Abstract
A new alternative rabies bait vaccine strain named ERAG3G, which is applicable to wild animals, was developed to eliminate rabies in South Korea. In this study, the safety and immunogenicity of the strain was evaluated in Korean raccoon dogs. The ERAG3G was propagated in BHK/T7-9 cells. Korean raccoon dogs were administered ERAG3G (1 ml, 108.0 FAID50/ml) orally or intramuscularly to evaluate its safety and immunogenicity. The raccoon dogs were observed for 70 days after administration, and immunogenicity was measured using a fluorescent antibody virus neutralization test. The ERAG3G strain was not pathogenic to Korean raccoon dogs immunized via the intramuscular or oral route. Raccoon dogs administered the candidate vaccine via the oral route developed high virus neutralizing antibody (VNA) titers ranging from 13.7 to 41.6 IU/ ml 70 days post administration. Raccoon dogs inoculated intramuscularly with the ERAG3G strain developed moderate VNA titers ranging from 0.5 to 13.7 IU/ ml. These findings suggest that the ERAG3G strain is safe and induces a protective immune response in raccoon dogs.
Go to : 
REFERENCES
1). Yousaf MZ, Qasim M, Zia S, Khan Mu, Ashfaq UA, Khan S. Rabies molecular virology, diagnosis, prevention and treatment. Virol J. 2012; 21(9):50.

2). Yang DK, Nakagawa K, Ito N, Kim HH, Hyun BH, Nah JJ, et al. A single immunization with recombinant rabies virus (ERAG3G) confers complete protection against rabies in mice. Clin Exp Vaccine Res. 2014; 3:176–84.

3). Oem JK, Kim SH, Kim YH, Lee MH, Lee KK. Reemergence of rabies in the southern Han river region, Korea. J Wildl Dis. 2014; 50:681–8.

4). Sobey KG, Rosatte R, Bachmann P, Buchanan T, Bruce L, Donovan D, et al. Field evaluation of an inactivated vaccine to control raccoon rabies in Ontario, Canada. J Wildl Dis. 2010; 46:818–31.

5). Hanlon CA, Niezgoda M, Morrill P, Rupprecht CE. Oral efficacy of an attenuated rabies virus vaccine in skunks and raccoons. J Wildl Dis. 2002; 38:420–7.

6). Orciari LA, Niezgoda M, Hanlon CA, Shaddock JH, Sanderlin DW, Yager PA, et al. Rapid clearance of SAG-2 rabies virus from dogs after oral vaccination. Vaccine. 2001; 19:4511–8.

7). Baer GM, Abelseth MK, Debbie JG. Oral vaccination of foxes against rabies. Am J Epidemiol. 1971; 93:487–90.

8). Brown LJ, Rosatte RC, Fehlner-Gardiner C, Bachmann P, Ellison JA, Jackson FR, et al. Oral vaccination and protection of red foxes (Vulpes vulpes) against rabies using ONRAB, an adenovirus-rabies recombinant vaccine. Vaccine. 2014; 32:984–9.

9). Faber M, Dietzschold B, Li J. Immunogenicity and safety of recombinant rabies viruses used for oral vaccination of stray dogs and wildlife. Zoonoses Public Health. 2009; 56:262–9.

10). Taylor J, Meignier B, Tartaglia J, Languet B, VanderHoeven J, Franchini G, et al. Biological and immunogenic properties of a canarypox-rabies recombinant, ALVAC-RG (vCP65) in non-avian species. Vaccine. 1995; 13:539–49.

11). Joo YS, Lee JH, Lee KK, Bang HA, Lee WC. Retrospective study of extensive vaccination programs for canine rabies control and public health in Korea. Jpn J Infect Dis. 2011; 64:513–5.
12). Rupprecht CE, Blass L, Smith K, Orciari LA, Niezgoda M, Whitfield SG, et al. Human infection due to recombinant vaccinia-rabies glycoprotein virus. N Engl J Med. 2001; 345:582–6.

13). Howard DR. Rabies virus titer from tissues of naturally infected skunks (Mephitis mephitis). Am J Vet Res. 1981; 42:1595–7.
14). Cliquet F, Aubert M, Sagné L. Development of a fluorescent antibody virus neutralisation test (FAVN test) for the quantitation of rabies-neutralising antibody. J Immunol Methods. 1998; 212:79–87.

15). Chiou HY, Hsieh CH, Jeng CR, Chan FT, Wang HY, Pang VF. Molecular characterization of cryptically circulating rabies virus from ferret badgers, Taiwan. Emerg Infect Dis. 2014; 20:790–8.

16). Yang DK, Kim SY, Oh YI, Lee JA, Cho SD, Lee KW, et al. Epidemiological Characteristics of Rabies in South Korea from January 2004 to March 2011. J Bacteriol Virol. 2011; 41:165–171.

17). Franka R, Wu X, Jackson FR, Velasco-Villa A, Palmer DP, Henderson H, et al. Rabies virus pathogenesis in relationship to intervention with inactivated and attenuated rabies vaccines. Vaccine. 2009; 27:7149–55.

18). Follmann EH, Ritter DG, Baer GM. Evaluation of the safety of two attenuated oral rabies vaccines, SAG1 and SAG2, in six Arctic mammals. Vaccine. 1996; 14:270–3.

19). Fehlner-Gardiner C, Rudd R, Donovan D, Slate D, Kempf L, Badcock J. Comparing ONRAB® AND RABORAL V-RG® oral rabies vaccine field performance in raccoons and striped skunks, New Brunswick, Canada, and Maine, USA. J Wildl Dis. 2012; 48:157–67.
20). Cliquet F, Aubert M. Elimination of terrestrial rabies in Western European countries. Dev Biol (Basel). 2004; 119:185–204.
21). Cliquet F, Gurbuxani JP, Pradhan HK, Pattnaik B, Patil SS, Regnault A, et al. The safety and efficacy of the oral rabies vaccine SAG2 in Indian stray dogs. Vaccine. 2007; 25:3409–18.

22). Tuffereau C, Leblois H, Bénéjean J, Coulon P, Lafay F, Flamand A. Arginine or lysine in position 333 of ERA and CVS glycoprotein is necessary for rabies virulence in adult mice. Virology. 1989; 172:206–12.

23). Morimoto K, Hooper DC, Spitsin S, Koprowski H, Dietzschold B. Pathogenicity of different rabies virus variants inversely correlates with apoptosis and rabies virus glycoprotein expression in infected primary neuron cultures. J Virol. 1999; 73:510–8.

24). Yang DK, Kim HH, Choi SS, Kim JT, Jeong WH, Song JY. Oral immunization of mice with recombinant rabies vaccine strain (ERAG3G) induces complete protection. Clin Exp Vaccine Res. 2015; 4:107–13.

25). Bankovskiy D, Safonov G, Kurilchuk Y. Immunogenicity of the ERA G333 rabies virus strain in foxes and raccoon dogs. Dev Biol (Basel). 2008; 131:461–6.
Go to : 
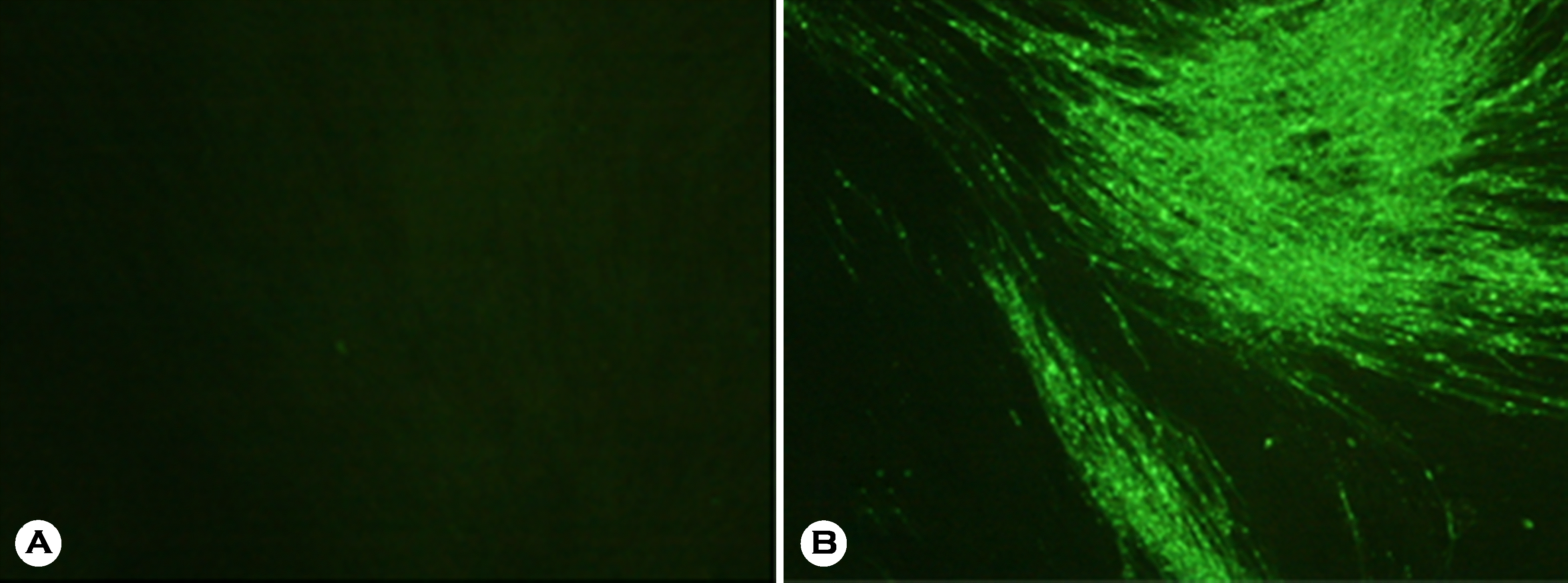 | Figure 1.Example of fluorescent assay virus neutralization (FAVN) test performed in BHK-21 cells. Fluorescent in the BHK-21 cells was not found in the A neutralized by serum antibody against RABV, but fluorescent appeared in the B due to absence of serum antibody against RABV. |
Table 1.
Virus neutralizing antibody (VNA) titers of raccoon dogs administrated with ERAG3G strain via oral or intramuscular route
| Group | Animal No. | Route of inoculation | VNA titer (IU/ ml) | ||
|---|---|---|---|---|---|
| 0 week | 5 weeks | 10 weeks | |||
| Group 1 | 1 | 0.07 | 41.6 | 41.6 | |
| 2 | Oral | 0.07 | 13.7 | 13.7 | |
| 3 | 0.07 | 13.7 | 13.7 | ||
| 4 | 0.07 | 7.90 | 13.7 | ||
| GM∗ | 0.07 | 19.2 | 20.6 | ||
| SD∗∗ | 0.00 | 15.2 | 14.0 | ||
| Group 2 | 5 | 0.07 | 0.87 | 0.50 | |
| 6 | IM | 0.07 | 0.87 | 0.5 | |
| 7 | 0.07 | 1.51 | 13.7 | ||
| 8 | 0.07 | 1.51 | 13.7 | ||
| GM | 0.07 | 1.19 | 7.10 | ||
| SD | 0.00 | 0.40 | 7.60 | ||
| Group 3 (control) | 9 10 | No treatment | 0.07 0.07 | 0.07 0.07 | 0.07 0.07 |
| GM | 0.07 | 0.07 | 0.07 | ||
| SD | 0.00 | 0.00 | 0.00 | ||




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download