Abstract
Getah virus (GETV), which is transmitted by mosquitoes, causes lower limb edema and stiffness in horses. In this study, we investigated the sero-surveillance of GETV among Thoroughbred racehorses in Korea during 2013 and 2014. A total of 1,182 equine serum samples collected from Thoroughbred racehorses in four provinces (Gyeongnam, Gyeonggi, Jeonbuk and Jeju provinces) were analyzed using virus neutralization (VN) tests. An antibody titer of ≥ 1:2 was considered positive. Overall, the seropositivity rate for GETV was found to be 12.4% (146/1,182) among the racehorses; the annual seropositivity rates were 12.4% and 12.2% in 2013 and 2014, respectively. The seropositivity rates in April and September in 2013 turned out to be 8.6% and 15.2%, respectively. The regional distribution of seropositivity ranged from 5.0% to 22.3% in 2013 and from 0.0% to 15.0% in 2014, respectively. Gyeongnam province had the highest seropositivity rate than other provinces. By analyzing the distribution of VN titers according to horse age, we found that the highest GETV seropositivity rate was in horses over 6 years of age (22.4% and 28.1%, 2013 and 2014, respectively), and that the incidence of GETV was higher in geldings (17.6% and 18.6%, 2013 and 2014, respectively) than in males and females. These results indicate that Thoroughbred horses raised in Korea were bitten by mosquitoes harboring GETV.
Go to : 
REFERENCES
1). Ministry of Agriculture and Forestry (MAF). Agricultural and Forestry Statistical Yearbook. MAF;Seoul: 2014.
2). Van Regenmortel MHV, Fauquet CM, Bishop DHL, Carstens EB, Estes MK, Lemon SM, et al. Virus taxonomy: Classification and nomenclature of viruses. 7 th Report of the International Committee on Taxonomy of Viruses. San Diego: Academic Press;2000. p. 884–7.
3). Calisher CH, Walton TE. Getah virus infection. Virus Infection of Equines. Amsterdam: Elsevier;1996. p. 157–65.
4). Calisher CH, Shope RE, Brandt W, Casals J, Karabatsos N, Murphy FA, et al. Proposed antigenic classification of registered arboviruses I. Togaviridae, Alphavirus. Intervirology. 1980; 14:229–32.
5). Pfeffer M, Kinney RM, Kaaden OR. The alphaviruse 3′-nontranslated region: size heterogeneity and arrangement of repeated sequence elements. Virology. 1998; 240:100–8.
6). Strauss JH, Strauss EG. The alphaviruses: Gene expression, replication, and evolution. Microbiol Rev. 1994; 58:491–562.

7). Berge TO. International catalogue of arboviruses, including certain other viruses of vertebrates. 2nd ed.Washington: US Department of Health, Education and Welfare;1975. p. 278–9.
8). Kumanomido T, Fukunaga Y, Ando Y, Kamada M, Imagawa H, Wada R, et al. Getah virus isolations from mosquitoes in an enzootic area in Japan. Nihon Juigaku Zasshi. 1986; 48:1135–40.

9). Li XD, Qiu FX, Yang H, Rao YN, Calisher CH. Isolation of Getah virus from mosquitoes collected on Hainan Island, China, and results of a serosurvey. Southeast Asian J Trop Med Public Health. 1992; 23:730–4.
10). Seo HJ, Kim HC, Klein TA, Park JY, Cho YS, Cho IS, et al. Characterization of recent Getah virus isolates from South Korea. Acta Virol. 2012; 56:265–7.

11). Doherty RL, Carley JG, Mackerras MJ, Marks EN. Studies of arthropod-borne virus infections in Queensland. III. Isolation and characterization of virus strains from wild-caught mosquitoes in North Queensland. Aust J Exp Biol Med Sci. 1963; 41:17–39.
12). Kamada M, Ando Y, Fukunaga Y, Kumanomido T, Imagawa H, Wada R, et al. Equine Getah virus infection: isolation of the virus from racehorses during an enzootic in Japan. Am J Trop Med Hyg. 1980; 29:984–8.

13). Brown CM, Timoney PJ. Getah virus infection of Indian horses. Trop Anim Health Prod. 1998; 30:241–52.
14). Shortridge KF, Mason DK, Watkins KL, Aaskov JG. Serological evidence for the transmission of Getah virus in Hong Kong. Vet Rec. 1994; 134:527–8.

15). Rhee YO, Heo Y, Kim YH, Sul DS. Serological survey of horses in Korea for evidence of Getah virus infection. Korean J Vet Res. 1986; 26:93–6.
16). Sugiyama I, Shimizu E, Nogami S, Suzuki K, Miura Y, Sentsui H. Serological Survey of Arthropod-Borne Viruses among Wild Boars in Japan. J Vet Med Sci. 2009; 71:1059–61.

17). Kumanomido T, Kamada M, Wada R, Kenemaru T, Sugiura T, Akiyama Y. Pathogenicity for horses of original Sagiyama virus, a member of the Getah virus group. Vet Microbiol. 1988; 17:367–73.

18). Kumanomido T, Wada R, Kanemaru T, Kamada M, Hirasawa K, Akiyama Y. Clinical and virological observations on swine experimentally infected with getah virus. Vet Microbiol. 1988; 16:295–301.

19). Kamada M, Kumanomido T, Wada R, Fukunaga Y, Imagawa H, Sugiura T. Intranasal infection of Getah virus in experimental horses. J Vet Med Sci. 1991; 53:855–8.

20). Sentsui H, Kono Y. An epidemic of Getah virus infection among racehorses: isolation of the virus. Res Vet Sci. 1980; 29:157–61.

21). Sentsui H, Kono Y. Reappearance of Getah virus infection among horses in Japan. Nihon Juigaku Zasshi. 1985; 47:333–5.

22). Chanas AC, Johnson BK, Simpson DIH. A Comparative Study of Related Alphaviruses a Naturally Occurring Model of Antigenic Variation in the Getah Sub-group. J Gen Virol. 1977; 35:455–62.
23). Lyoo YS, Chang CH, Rhee JC, Kim YH, Lee SY. Isolation of Getah virus from racehorse in Korea. Korean J Vet Res. 1991; 31:189–94.
24). Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Epidemiol. 1938; 27:493–7.
25). Tesh RB, McLean RG, Shroyer DA, Calisher CH, Rosen L. Ross River virus (Togaviridae: Alphavirus) infection (epidemic polyarthritis) in American Samoa. Trans R Soc Trop Med Hyg. 1981; 75:426–31.

26). Cheun HI, Cho SH, Lee HI, Shin EH, Lee JS, Kim TS, et al. Seasonal prevalence of mosquitoes, including vectors of Brugian filariasis, in southern islands of the Republic of Korea. Korean J Parasitol. 2011; 49:59–64.
27). Sucharit S, Surathin K, Shrestha SR. Vectors of Japanese encephalitis virus (JEV): species complexes of the vectors. Southeast Asian J Trop Med Public Health. 1989; 20:611–21.
28). Regional Vector Surveillance Center for Climate Change (Yeongnam). Research report, Korea Centers For Disease Control and Prevention (KCDC). 2011. http://academic.naver.com/view.nhn?dir_id=2&unFold=false&sort=0&query=%EA%B8%88%EB%B9%9B%EC%88%B2%EB%AA%A8%EA%B8%B0&gk_qvt=0&citedSearch=false&field=0&gk_adt=0&qvt=1&doc_id=57487065&page.page=1&ndsCategoryId=207.
Go to : 
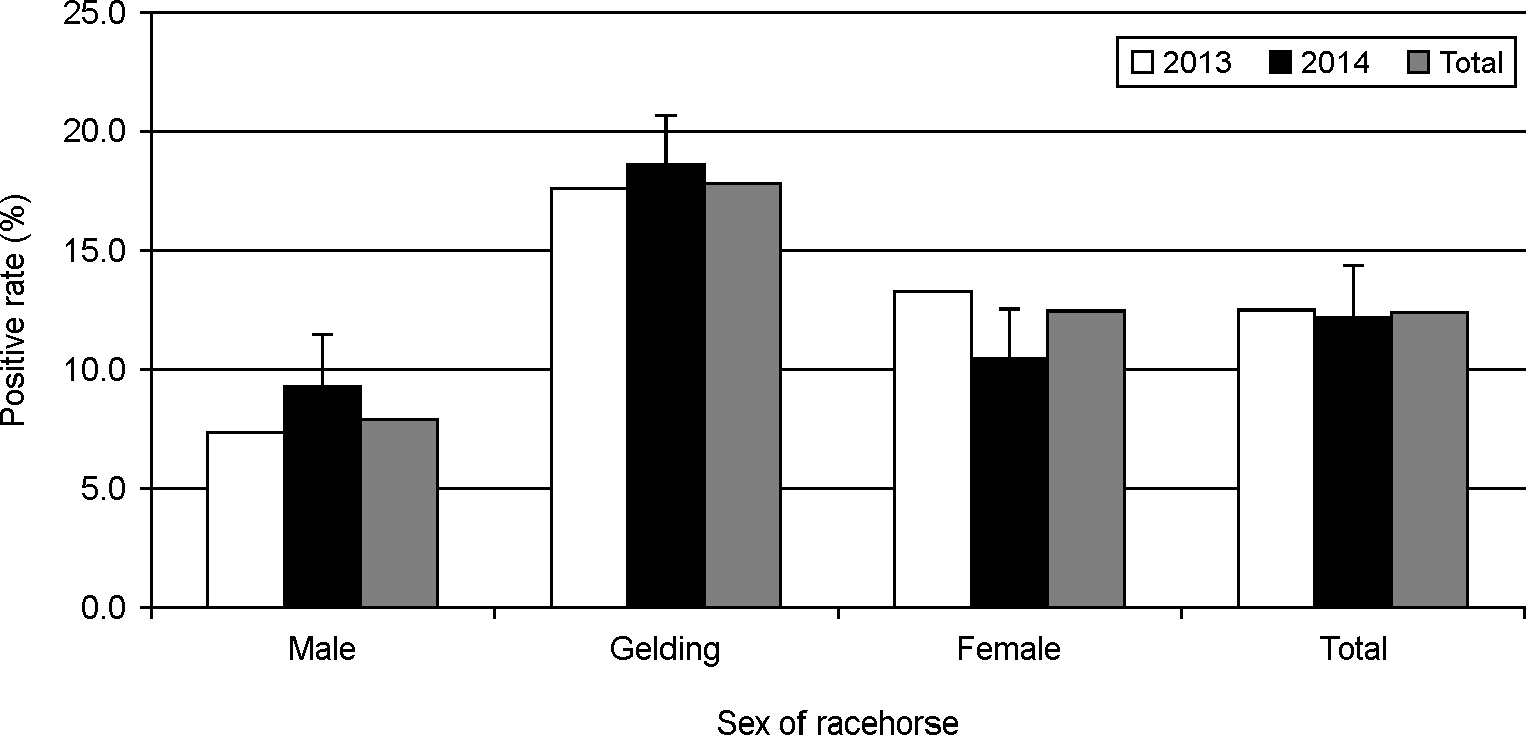 | Figure 1.Seropositivity rates for GETV gender among 1,182 Korean Thoroughbred horses between 2013 and 2014. |
Table 1.
Distribution of virus neutralizing titers against GETV among Korean Thoroughbred horses between 2013 and 2014
Table 2.
Seasonal seropositivity rate of the Korean Thoroughbred horses in 2013
| Month of collection | No. of samples | No. of positive | Seropositivity rates (%) | No. of the same samples | No. of positive | No. of high titer |
|---|---|---|---|---|---|---|
| April | 348 | 30 | 8.6 | 47 | 13 | 2 |
| September | 474 | 72 | 15.2 | 11 |
Table 3.
Regional distribution of GETV antibodies among Koran Thoroughbred horses between 2013 and 2014
| 2013 | 2014 | ||||||
|---|---|---|---|---|---|---|---|
| Province | No. of samples | No. of positive | Sero-positivity rates (%) | No. of samples | No. of positive | Sero-positivity rates (%) | Total |
| GG∗ | 378 | 32 | 8.5 | 130 | 17 | 13 | 9.6 |
| GN | 175 | 39 | 22.3 | 100 | 15 | 15 | 19.6 |
| JB | 40 | 2 | 5.0 | 20 | 0 | 0 | 3.3 |
| JJ | 229 | 29 | 12.7 | 110 | 12 | 10.9 | 12.1 |
| Total | 822 | 102 | 12.4 | 360 | 44 | 12.2 | 12.4 |
Table 4.
Distribution of seropositivity rates according to horse between 2013 and 2014




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download