Abstract
Naturally occurring reoviruses are live replication-proficient viruses specifically infecting human cancer cell while sparing normal counterpart. Since the discovery of reoviruses in 1950s, reoviruses have shown various degrees of safety and efficacy in pre-clinical or clinical application for human anti-cancer therapeutics. I have recently shown that cellular tumor suppressor genes, such as p53, ATM (Ataxia telangiectasia mutated), and RB (Retinoblastoma associated), are important in determining reoviral oncotropism. Thus, it is interesting to examine whether the aberrancy of c-Myc expression, whose normal function also plays an important role in the maintenance of genomic integrity, could affect reoviral oncolytic tropism. Hs68 cells are non-tumorigenic normal cells and resistant to reoviral cytopathic effects. Importantly, I found that c-Myc overexpression in human HS68 cells effectively induced reovirus cytophatic effects compared to mock expressed cells as shown by the typical reoviral cytophathology and an increased level of caspase-3 activity. Taken together, overexpression of c-Myc could play an important role in determining reoviral oncolytic tropism.
Go to : 
REFERENCES
1). Tyler KL. Mammalian reoviruses. Fields BN, Knipe DM, Howley M, editors. Fields Virology. Philadelphia, USA: Lippincott-Raven;2001. p. 1729–45.
2). Ramig RF, Cross RK, Fields BN. Genome RNAs and polypeptides of reovirus serotypes 1, 2, and 3. J Virol. 1977; 22:726–33.

3). Stoeckel J, Hay JG. Drug evaluation: Reolysin–wild- type reovirus as a cancer therapeutic. Curr Opin Mol Ther. 2006; 8:249–60.
5). Roberts MS, Lorence RM, Groene WS, Bamat MK. Naturally oncolytic viruses. Curr Opin Mol Ther. 2006; 8:314–21.
6). Wang G, Barrett JW, Stanford M, Werden SJ, Johnston JB, Gao X, et al. Infection of human cancer cells with myxoma virus requires Akt activation via interaction with a viral ankyrin-repeat host range factor. Proc Natl Acad Sci U S A. 2006; 103:4640–5.

7). Strong JE, Coffey MC, Tang D, Sabinin P, Lee PW. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO J. 1998; 17:3351–62.

9). Prochownik EV, Li Y. The ever expanding role for c-Myc in promoting genomic instability. Cell Cycle. 2007; 6:1024–9.

10). Prochownik EV. c-Myc: linking transformation and genomic instability. Curr Mol Med. 2008; 8:446–58.

11). Coschi CH, Dick FA. Chromosome instability and deregulated proliferation: an unavoidable duo. Cell Mol Life Sci. 2012; 69:2009–24.

12). Kim M, Williamson CT, Prudhomme J, Bebb DG, Riabowol K, Lee PW, et al. The viral tropism of two distinct oncolytic viruses, reovirus and myxoma virus, is modulated by cellular tumor suppressor gene status. Oncogene. 2010; 29:3990–6.

13). Kim M, Egan C, Alain T, Urbanski SJ, Lee PW, Forsyth PA, et al. Acquired resistance to reoviral oncolysis in Ras-transformed fibrosarcoma cells. Oncogene. 2007; 26:4124–34.

14). Nieminen AI, Partanen JI, Klefstrom J. c-Myc blazing a trail of death: coupling of the mitochondrial and death receptor apoptosis pathways by c-Myc. Cell Cycle. 2007; 6:2464–72.

15). Gabay M, Li Y, Felsher DW. MYC activation is a hallmark of cancer initiation and maintenance. Cold Spring Harb Perspect Med. 2014; 4.

17). Spandidos DA, Dokianakis DN, Kallergi G, Aggelakis E. Molecular basis of gynecological cancer. Ann N Y Acad Sci. 2000; 900:56–64.

18). Aunoble B, Sanches R, Didier E, Bignon YJ. Major oncogenes and tumor suppressor genes involved in epithelial ovarian cancer (review). Int J Oncol. 2000; 16:567–76.

19). Hirasawa K, Nishikawa SG, Norman KL, Alain T, Kossakowska A, Lee PW. Oncolytic reovirus against ovarian and colon cancer. Cancer Res. 2002; 62:1696–701.
20). ClinicalTrials.gov. [homepage on the Internet]. NCT- 01199263: Paclitaxel With or Without Viral Therapy in Treating Patients With Recurrent or Persistent Ovarian Epithelial, Fallopian Tube, or Primary Peritoneal Cancer. Available from:. www.clinicaltrials.gov/ct2/show/NCT-01199263.
21). ClinicalTrials.gov. [homepage on the Internet]. NCT- 00602277: Viral Therapy in Treating Patients With Ovarian Epithelial Cancer, Primary Peritoneal Cancer, or Fallopian Tube Cancer That Did Not Respond to Platinum Chemotherapy. Available from:. www.clinicaltrials.gov/ct2/show/NCT00602277.
Go to : 
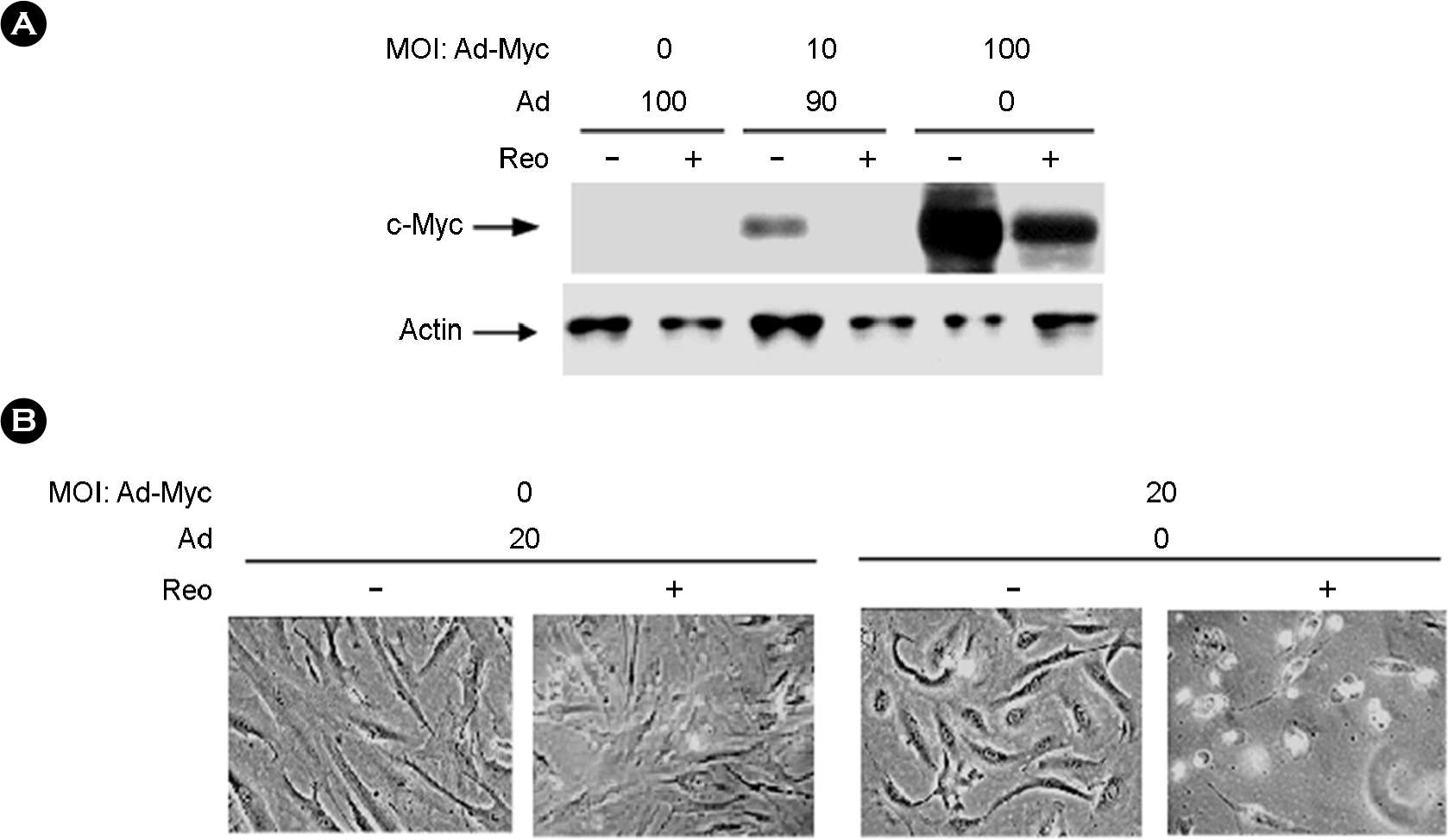 | Figure 1.c-Myc overexpressing HS68 cells and reovirus induced cytopathology. (A) HS68 cells were infected with an Ad-Myc or an Ad-control vector at the MOIs appropriate to make the total MOI of adenovirus up to 100 pfu/cell on each plate, were allowed to accumulate c-Myc protein for 24 hours and then were either infected with reovirus at 20 MOI, or were mock infected. Cells were lysed 48 hours post-reovirus infection, lysed and 25 μg of whole cell lysate was separated on a 12% acrylamide gel. Gels were western blotted against c-Myc and actin proteins. (B) HS68 cells were infected with the Ad-Myc vector at MOIs indicated and infected with the Ad-control vector at a MOI. appropriate to make the total MOI of adenovirus up to 20 pfu/cell on each plate. Cells were allowed to accumulate c-Myc proteins for 12 hours prior to being infected or mock-infected with reovirus. Photos of the cells were taken Mock infection and at 48 hours post-reovirus infection at 20 MOI. |
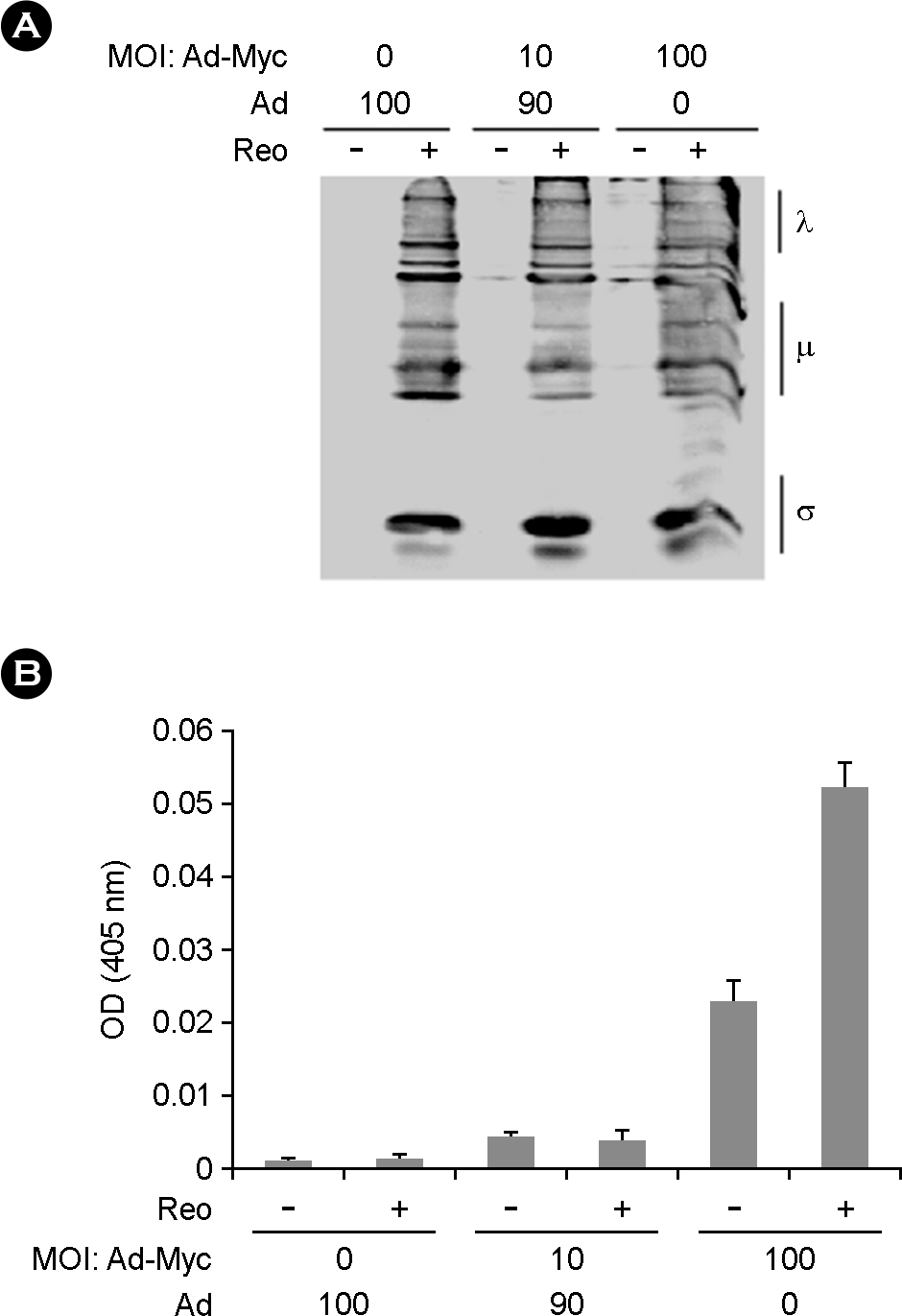 | Figure 2.Caspase 3 activity of c-Myc expressing HS68 cells upon reovirus exposure. (A) HS68 cells were infected with the Ad-Myc or Ad-control vectors at MOIs indicated and allowed to accumulate c-Myc protein for 24 hours before infecting with reovirus or mock-infecting. Cells were harvested at 48 hours post-reovirus infection, lysed and 25 μg of cell lysate was probed against reoviral structural proteins. (B) HS68 cells were treated as indicated and given 12 hours to accumulate c-Myc protein prior to reovirus infection. Cells were harvested 24 hours post-reovirus infection (MOI of 20), lysed, and caspase-3 activity was measured as described in Materials and Methods. Activity was determined colorimetrically by absorbance at 405 nm. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download