Abstract
Bovine tuberculosis caused by Mycobacterium bovis is a major economic problem in several countries. Antibody responses are useful indicators of M. bovis infection of cattle. To overcome drawback of serological tests with low sensitivity, identification and characterization of multiple serodiagnostic antigens has been required. In this study, the antigens with strong antibody reactivity were searched using fractionation of M. bovis culture filtrate proteins and probing with sera from M. bovis-infected cattle. Twelve proteins which have not previously been described as serologic targets were identified and six proteins among them were expressed in Escherichia coli. The mycobacterial lipoarabinomannan (LAM) with strong seroreactivity in cattle was identified and purified. IgG and IgA responses against the newly identified proteins, the seroreactive proteins with strong antibody reactivity in human tuberculosis, and LAM were compared in M. bovis-infected and non-infected cattle as well as in field samples. In general, sensitivity of the tested antigens was higher in M. bovis-infected cattle than purified protein derivative (PPD) (+) field samples. Although a diverse reactivity and sensitivity according to the antigens were shown, the diagnostic utility of both IgA and IgG antibody to the antigens was similar in M. bovis-infected cattle but utility of IgG antibody was superior to that of IgA in field samples. The antigen with the highest diagnostic value was LAM in both the groups. Other antigens with considerable diagnostic utility were BCG_3488c, BCG_2330, Antigen 85, HspX, and Rv3593 when considered the sensitivity and area under the receiver characteristic curve (AUC) value. These antigens may be valuable candidates to be included in a cocktail test kit for bovine tuberculosis diagnosis.
Go to : 
REFERENCES
1). Vordermeier HM, Chambers MA, Buddle BM, Pollock JM, Hewinson RG. Progress in the development of vaccines and diagnostic reagents to control tuberculosis in cattle. Vet J. 2006; 171:229–44.

2). Neill SD, Cassidy J, Hanna J, Mackie DP, Pollock JM, Clements A, et al. Detection of Mycobacterium bovis infection in skin test-negative cattle with an assay for bovine interferon-gamma. Vet Rec. 1994; 135:134–5.
3). Wadhwa A, Johonson RE, Eda K, Waters WR, Palmer MV, Bannantine JP, et al. Evaluation of ethanol vortex ELISA for detection of bovine tuberculosis in cattle and deer. BMC Vet Res. 2014; 10:147.

4). Waters WR, Buddle BM, Vordermeier HM, Gormley E, Palmer MV, Thacker TC, et al. Development and evaluation of an enzyme-linked immunosorbent assay for use in the detection of bovine tuberculosis in cattle. Clin Vaccine Immunol. 2011; 18:1882–8.

5). Schiller I, Oesch B, Vordermeier HM, Palmer MV, Harris BN, Orloski KA, et al. Bovine tuberculosis: a review of current and emerging diagnostic techniques in view of their relevance for disease control and eradication. Transbound Emerg Dis. 2010; 57:205–20.

6). Souza II, Melo ES, Ramos CA, Farias TA, Osório AL, Jorge KS, et al. Screening of recombinant proteins as antigens in indirect ELISA for diagnosis of bovine tuberculosis. Springerplus. 2012; 1:77.

7). Casal C, Díez-Guerrier A, Álvarez J, Rodriguez-Campos S, Mateos A, Linscott R, et al. Strategic use of serology for the diagnosis of bovine tuberculosis after intradermal skin testing. Vet Microbiol. 2014; 170:342–51.

8). Wadhwa A, Hickling GJ, Eda S. Opportunities for improved serodiagnosis of human tuberculosis, bovine tuberculosis, and paratuberculosis. Vet Med Int. 2012; 2012:674238.

9). Fifis T, Rothel JS, Wood PR. Soluble Mycobacterium bovis protein antigens: studies on their purification and immunological evaluation. Vet Microbiol. 1994; 40:65–81.
10). Jeon HS, Shin AR, Son YJ, Kim JM, Jang Y, Kim S, et al. An evaluation of the use of immunoglobulin A antibody response against mycobacterial antigens for the diagnosis of Mycobacterium bovis infection in cattle. J Vet Diagn Invest. 2015.
11). Byun EH, Kim WS, Shin AR, Kim JS, Whang J, Won CJ, et al. Rv0315, a novel immunostimulatory antigen of Mycobacterium tuberculosis, activates dendritic cells and drives Th1 immune responses. J Mol Med. 2012; 90:285–98.
12). Hamasur B, Källenius G, Svenson SB. A new rapid and simple method for large-scale purification of mycobacterial lipoarabinomannan. FEMS Immunol Med Microbiol. 1999; 24:11–7.

13). Mazurek J, Ignatowicz L, Kallenius G, Svenson SB, Pawlowski A, Hamasur B. Divergent effects of mycobacterial cell wall glycolipids on maturation and function of human monocyte-derived dendritic cells. PLoS One. 2012; 7:e42515.

14). Kwon YM, Jung KH, Choi GE, Shin AR, Lee BS, Won CJ, et al. Identification and diagnostic utility of serologic reactive antigens from Mycobacterium tuberculosis sonic extracts. J Bacteriol Virol. 2009; 39:329–36.
15). Lee JS, Jo EK, Noh YK, Shin AR, Shin DM, Son JW, et al. Diagnosis of pulmonary tuberculosis using MTB12 and 38-kDa antigens. Respirology. 2008; 13:432–7.

16). Shin AR, Shin SJ, Lee KS, Eom SH, Lee SS, Lee BS, et al. Improved sensitivity of diagnosis of tuberculosis in patients in Korea via a cocktail enzyme-linked immunosorbent assay containing the abundantly expressed antigens of the K strain of Mycobacterium tuberculosis. Clin Vaccine Immunol. 2008; 15:1788–95.
17). Choi GE, Eom SH, Jung KH, Son JW, Shin AR, Shin SJ, et al. CysA2: A candidate serodiagnostic marker for Mycobacterium tuberculosis infection. Respirology. 2010; 15:636–42.
18). Organization WH. Global tuberculosis control: WHO report 2010. World Health Organization;2010.
19). Chambers MA. Review of the diagnosis and study of tuberculosis in non-bovine wildlife species using immunological methods. Transbound Emerg Dis. 2009; 56:215–27.

20). Shin AR, Lee KS, Lee JS, Kim SY, Song CH, Jung SB, et al. Mycobacterium tuberculosis HBHA protein reacts strongly with the serum immunoglobulin M of tuberculosis patients. Clin Vaccine Immunol. 2006; 13:869–75.
Go to : 
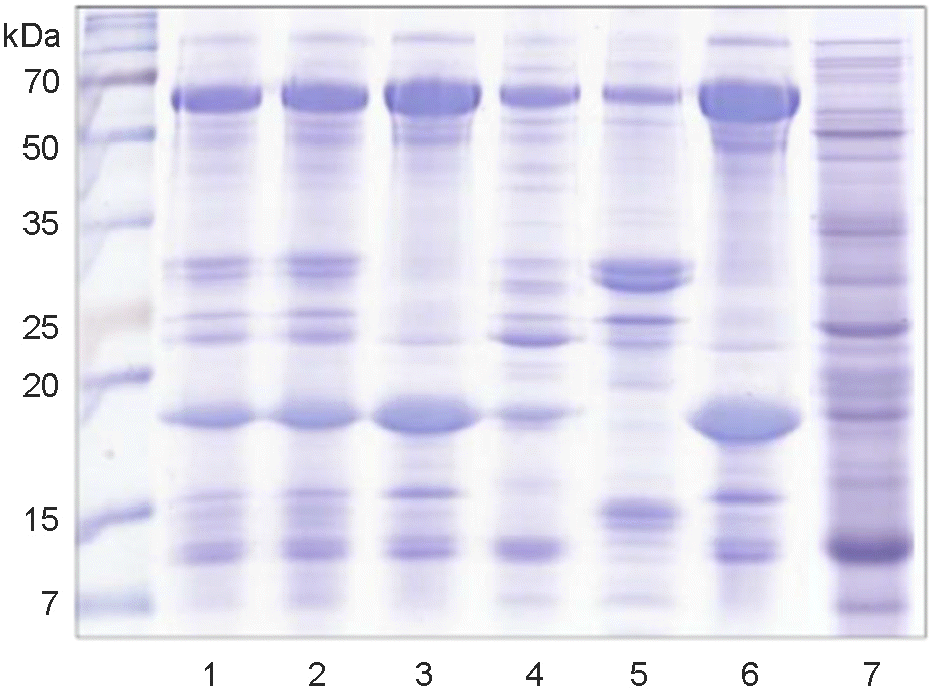 | Figure 1.
SDS-PAGE analysis of the fractionation of Mycobacterium bovis culture filtrate proteins (CFPs). The 0~80% or 50~80% ammonium sulfate precipitate (ASP) of M. bovis AN5 total CFPs (lane 1) was fractionated into a pass fraction, 50 mM potassium phosphate buffer (kPB) eluate, and 1 mM kPB eluate by hydrophobic interaction chromatography (HIC). Three fractions were separated into a pass fraction and 500 mM kPB eluate by hydroxylapatite (HAT) chromatography, and then if necessary, further fractionated by diethylaminoethanol (DEAE) ion exchange chromatography. Total CFPs → 0~80% ASP (lane 2), total CFPs → 0~80% ASP → HIC pass (lane 3), total CFPs → 0~80% ASP → HIC 50 mM (lane 4), total CFPs → 0~80% ASP → HIC 1 mM (lane 5), total CFPs → 0~80% ASP → HIC pass → HAT pass (lane 6), M. bovis BCG total CFPs → 50~80% ASP → HAT pass → DAEA 51~66 (lane 7). The gel was stained with Coomassie blue. |
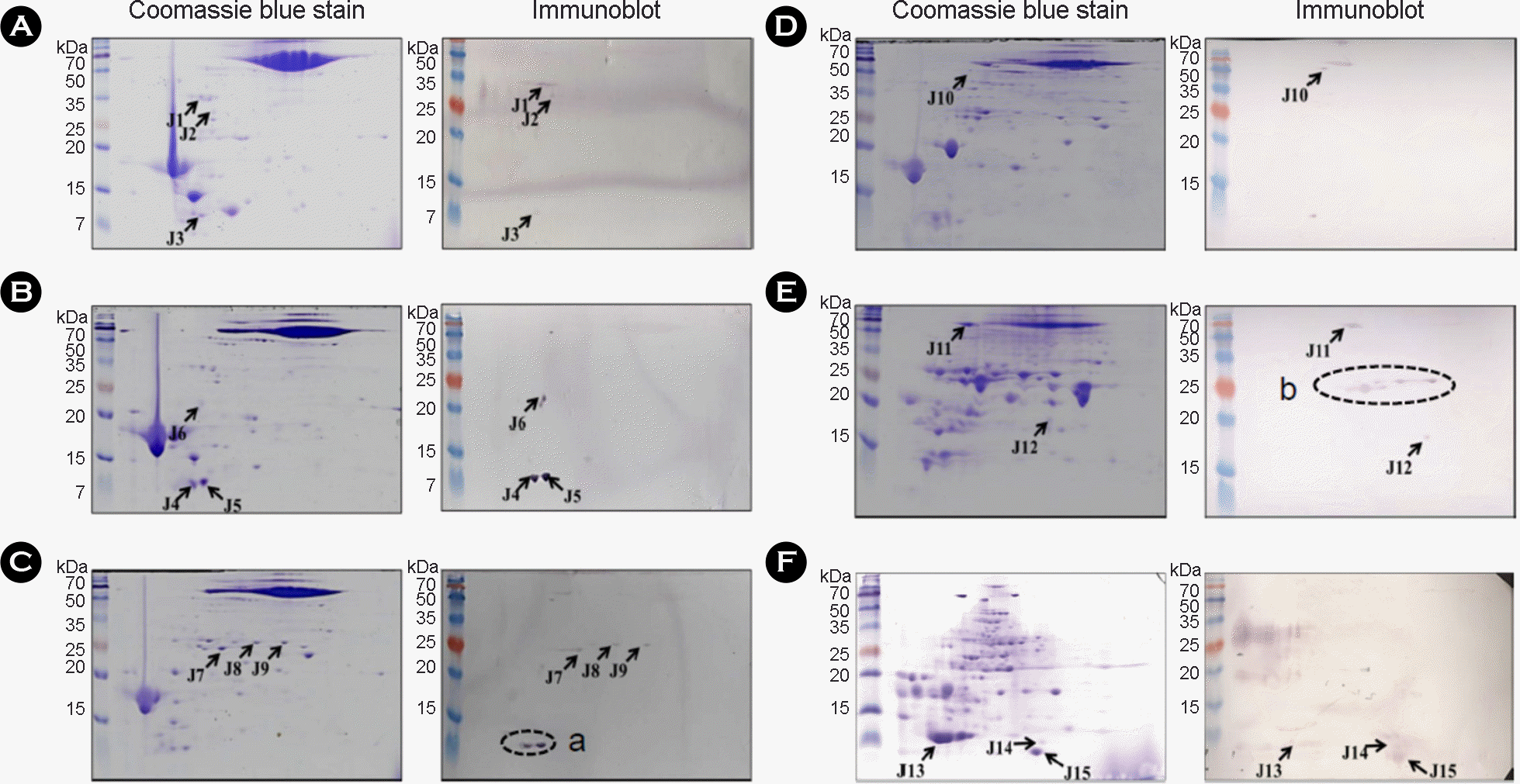 | Figure 2.
2-DE and immunoblot analysis of fractionated culture filtrate proteins (CFPs). The fractions were separated by isoelectric focusing using a 7 cm pH gradient strip (pH 4 to 7) in the first dimension, and 15% SDS-PAGE in the second dimension. M. bovis AN5 total CFPs → 0~80% ASP → HIC pass → HAT pass (A), total CFPs → 0~80% ASP → HIC pass (B), total CFPs → 0~80% ASP (C), total CFPs → 0~80% ASP → HIC 50 mM (D), total CFPs → 0~80% ASP → HIC 1 mM (E) and M. bovis BCG total CFPs →50~80% ASP → HAT pass → DAEA 51~66 (F). The gels were analyzed by Coomassie blue stain and immunoblot with M. bovis-infected sera. |
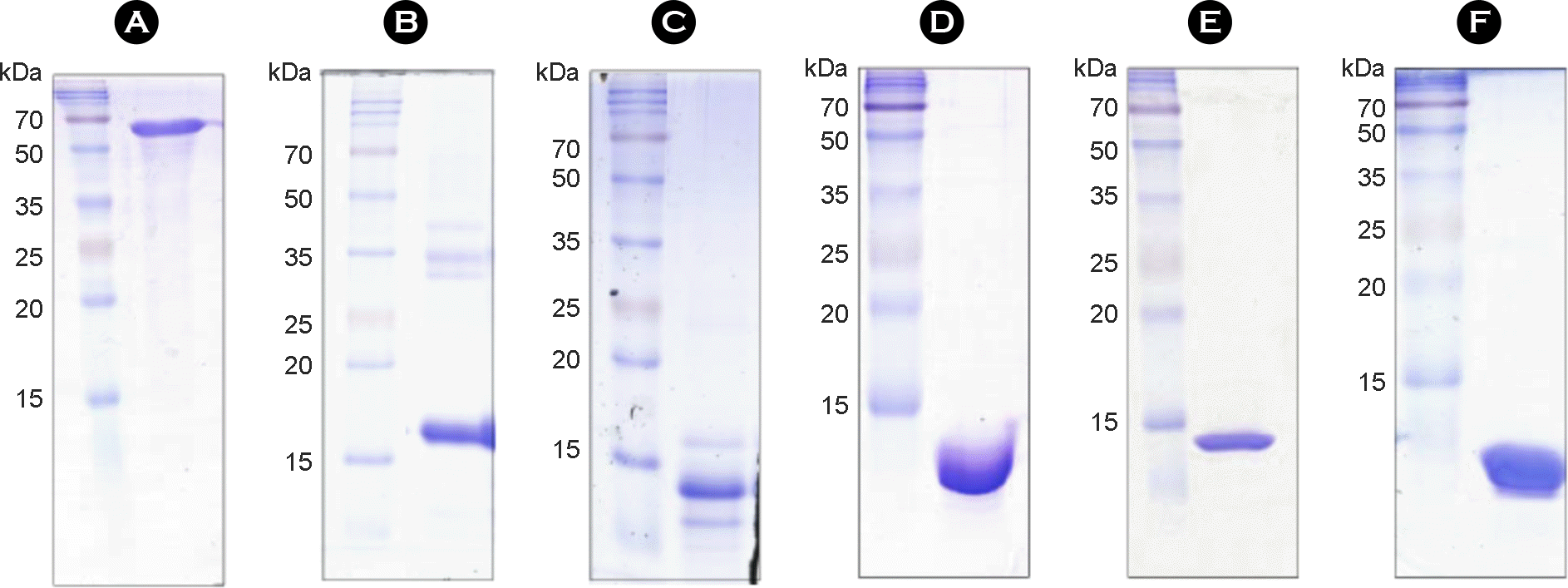 | Figure 3.
SDS-PAGE analysis of purified proteins. BCG_0389 (A), BCG_1909 (B), BCG_2330 (C), BCG_2765 (D), BCG_3488c (E) and BCG_3706c (F) proteins were overexpressed in Escherichia coli, purified by Ni-NTA affinity chromatography, and analyzed by SDS-PAGE with Coomassie blue staining. |
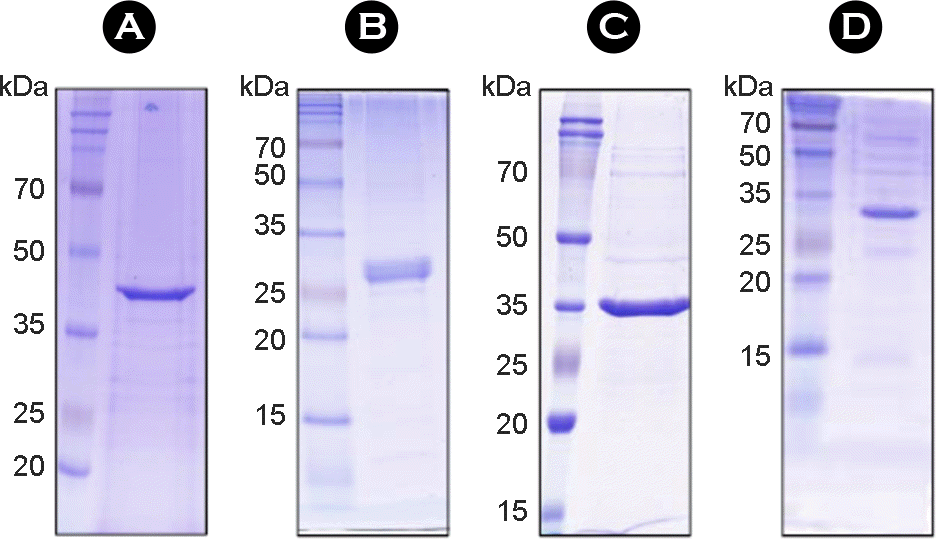 | Figure 4.
SDS-PAGE analysis of purified proteins with diagnostic utility in human tuberculosis. The purified recombinant proteins of Rv3593 (A), Rv1605 (B), MAV5183 (C) and MAV4300(D) were subjected to SDS-PAGE and stained with Coomassie blue. |
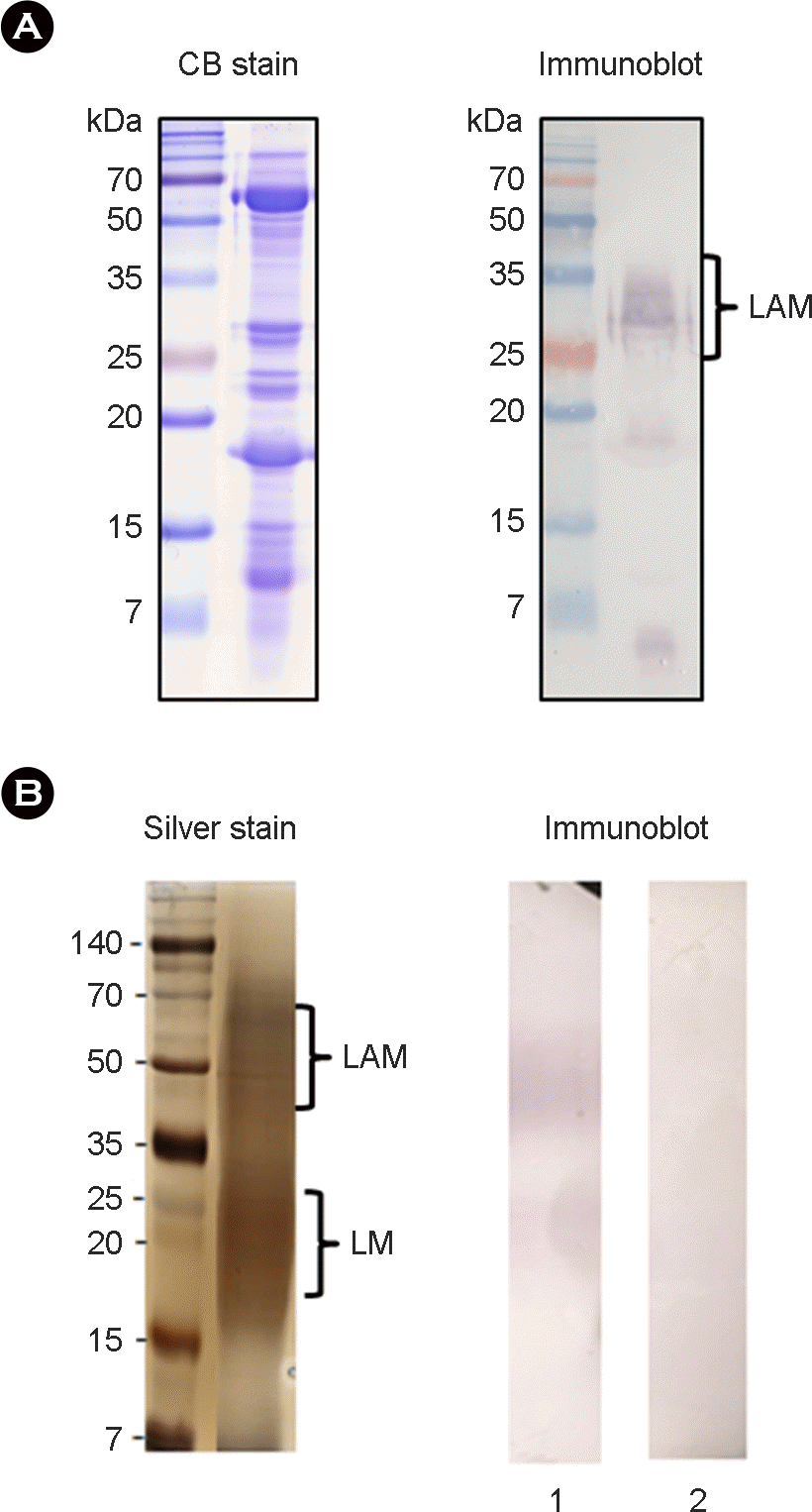 | Figure 5.
Antibody reactivity and purification of lipoarabinomannan (LAM). (A) The M. bovis CFPs was analyzed with Coomassie blue (CB) staining and immunoblot using M. bovis-infected cattle sera. (B) LAM from total lipid extract of M. tuberculosis was purified by Triton-X 114 phase partitioning, separated by SDS-PAGE, and then analyzed with silver staining and immunoblot using sera of M. bovis-infected (lane 1) or M. avium-infected cattle (lane 2). LM, lipomannan. |
Table 1.
Primers sets for genes amplified by polymerase chain reaction (PCR)
Table 2.
Identification of Mycobacterium bovis seroreactive proteins by liquid chromatography-electrospray ionization-mass spectrometry (LC-ESI-MS)
Table 3.
IgG antibody responses to each antigen in sera from Mycobacterium bovis-infected cattle and non-infected cattlea
| Antigens | Groups (n=60) | |||||
|---|---|---|---|---|---|---|
| M. bovis-infected cattle (n=30) | Non-infected cattle (n=30) | |||||
| Mean OD ± SD | % sensitivity (no. of positive samples) | AUC (μg · h/ml) (95% CI) | P valueb | Mean OD ± SD | % specificity (no. of positive samples) | |
| BCG_0389 | 0.0218±0.0206 | 23.3 (7) | 0.6422 (0.5007~0.7838) | 0.0585 | 0.0124±0.0132 | 90.0 (3) |
| BCG_2330 | 0.1664±0.0657 | 3.3 (1) | 0.6289 (0.4854~0.7724) | 0.0864 | 0.1252±0.092 | 100.0 (0) |
| BCG_2765 | 0.0021±0.0129 | 16.7 (5) | 0.6778 (0.5395~0.8160) | 0.018 | 0.0001±0.0094 | 96.7 (1) |
| BCG_3488c | 0.0065±0.0102 | 3.3 (1) | 0.7489 (0.6226~0.8752) | 0.0009 | 0.0018±0.012 | 96.7 (1) |
| Ag85 | 0.0738±0.2035 | 53.3 (16) | 0.8450 (0.7487~0.9413) | <0.0001 | 0.0002±0.0058 | 96.7 (1) |
| HspX | 0.0613±0.2137 | 26.7 (8) | 0.7711 (0.6531~0.8891) | 0.0003 | 0.0061±0.0082 | 93.3 (2) |
| CysA | 0.0137±0.0129 | 40.0 (12) | 0.8689 (0.7764~0.9613) | <0.0001 | 0.0017±0.0057 | 96.7 (1) |
| Rv1605 | 0.0076±0.0133 | 30.0 (9) | 0.7294 (0.5980~0.8609) | 0.0023 | 0.0002±0.0054 | 96.7 (1) |
| Rv3593 | 0.0229±0.0538 | 33.3 (10) | 0.7206 (0.5895~0.8516) | 0.0034 | 0.003±0.0059 | 96.7 (1) |
| MAV5183 | 0.2726±0.2444 | 6.7 (2) | 0.6178 (0.4736~0.7620) | 0.1171 | 0.3199±0.1582 | 100.0 (0) |
| MAV4300 | 0.0365±0.0351 | 10.0 (3) | 0.5550 (0.4072~0.7028) | 0.4643 | 0.0306±0.0278 | 93.3 (2) |
| LAM | 0.2118±0.2713 | 90.0 (27) | 0.9600 (00.9051~1.015) | <0.0001 | 0.0105±0.007 | 96.7 (1) |
Table 4.
IgA antibody responses to each antigen in sera from Mycobacterium bovis-infected cattle and non-infected cattlea
| Antigens | Groups (n=60) | |||||
|---|---|---|---|---|---|---|
| M. bovis-infected cattle (n=30) | Non-infected cattle (n=30) | |||||
| Mean OD ± SD | % sensitivity (no. of positive samples) | AUC (μg · h/ml) (95% CI) | P valueb | Mean OD ± SD | % specificity (no. of positive samples) | |
| BCG_0389 | 0.1447±0.2052 | 20.0 (6) | 0.6889 (0.5542~0.8235) | 0.012 | 0.0735±0.0448 | 96.7 (1) |
| BCG_2330 | 0.0722±0.2118 | 33.3 (10) | 0.7833 (0.6704~0.8963) | 0.0002 | 0.012±0.0105 | 96.7 (1) |
| BCG_2765 | 0.0625±0.0686 | 20.0 (6) | 0.6272 (0.4852~0.7692) | 0.0906 | 0.0331±0.0344 | 96.7 (1) |
| BCG_3488c | 0.0792±0.2108 | 50.0 (15) | 0.7856 (0.6702~0.9009) | 0.0001 | 0.0149±0.0098 | 100.0 (0) |
| Ag85 | 0.0808±0.2209 | 40.0 (12) | 0.7767 (0.6578~0.8956) | 0.0002 | 0.0075±0.0102 | 93.3 (2) |
| HspX | 0.0252±0.0225 | 10.0 (3) | 0.6672 (0.5281~0.8063) | 0.0261 | 0.0161±0.0209 | 90.0 (3) |
| CysA | 0.0083±0.0263 | 10.0 (3) | 0.7189 (0.5841~0.8537) | 0.0036 | 0.0001±0.0213 | 96.7 (1) |
| Rv1605 | 0.0973±0.2102 | 36.7 (11) | 0.7767 (0.6610~0.8923) | 0.0002 | 0.0233±0.0166 | 100.0 (0) |
| Rv3593 | 0.0507±0.041 | 40.0 (12) | 0.7883 (0.6759~0.9007) | 0.0001 | 0.0192±0.0137 | 96.7 (1) |
| MAV5183 | 0.2564±0.2707 | 20.0 (6) | 0.5633 (0.4160~0.7107) | 0.3994 | 0.1774±0.0881 | 96.7 (1) |
| MAV4300 | 0.1079±0.2081 | 30.0 (9) | 0.7667 (0.6485~0.8849) | 0.0004 | 0.0286±0.0349 | 96.7 (1) |
| LAM | 0.2164±0.3078 | 60.0 (18) | 0.8856 (0.8032~0.9679) | <0.0001 | 0.0247±0.0213 | 93.3 (2) |
Table 5.
IgG antibody responses to each antigen in field samplesa
| Antigens | Group (n=155) | |||||
|---|---|---|---|---|---|---|
| PPD (+) cattle (n=59) | PD (-) cattle (n=96) | |||||
| P Mean OD ± SD | % sensitivity (no. of positive samples) | AUC (μg · h/ml) (95% CI) | P valueb | P Mean OD ± SD | % specificity (no. of positive samples) | |
| BCG_0389 | 0.0253±0.0258 | 5.1 (3) | 0.5280 (0.4277~0.6284) | 0.56 | 0.0225±0.0224 | 96.9 (3) |
| BCG_2330 | 0.0806±0.0648 | 18.6 (11) | 0.6245 (0.5316~0.7174) | 0.0097 | 0.0514±0.0448 | 95.8 (4) |
| BCG_2765 | 0.0033±0.061 | 0.0 (0) | 0.5580 (0.4444~0.6715) | 0.2282 | 0.0021±0.0307 | 99.0 (1) |
| BCG_3488c | 0.0191±0.0197 | 55.9 (33) | 0.6956 (0.5989~0.7924) | <0.0001 | 0.0026±0.0073 | 95.8 (4) |
| Ag85 | 0.0072±0.014 | 13.6 (8) | 0.6724 0.5867~0.7581) | 0.0003 | 0.0011±0.0088 | 94.8 (5) |
| HspX | 0.049±0.2823 | 10.2 (6) | 0.5927 (0.5039~0.6815) | 0.0531 | 0.0099±0.0152 | 95.8 (4) |
| CysA | 0.0077±0.0062 | 1.7 (1) | 0.7071 (0.6263~0.7879) | <0.0001 | 0.0042±0.0113 | 96.9 (3) |
| Rv1605 | 0.0034±0.0061 | 0.0 (0) | 0.6326 (0.5454~0.7198) | 0.0057 | 0.0086±0.0114 | 93.7 (6) |
| Rv3593 | 0.0025±0.0092 | 8.5 (5) | 0.5535 (0.4608~0.6462) | 0.2665 | 0.0028±0.0068 | 96.9 (3) |
| MAV5183 | 0.4782±0.425 | 33.9 (20) | 0.6613 (0.5597~0.7629) | 0.0008 | 0.2652±0.2073 | 94.8 (5) |
| MAV4300 | 0.0326±0.03 | 8.5 (5) | 0.5767 (0.4849~0.6685) | 0.1108 | 0.0262±0.0271 | 94.8 (5) |
| LAM | 0.2129±0.2007 | 71.2 (42) | 0.9328 (0.8915~0.9740) | <0.0001 | 0.019±0.0206 | 96.9 (3) |
Table 6.
IgA antibody responses to each antigen in field samplesa
| Antigens | Group (n=155) | |||||
|---|---|---|---|---|---|---|
| PPD (+) cattle (n=59) | PPD (-) cattle (n=96) | |||||
| Mean OD ± SD | % sensitivity (no. of positive samples) | AUC (μg · h/ml) (95% CI) | P valueb | Mean OD ± SD | % specificity (no. of positive samples) | |
| BCG_0389 | 0.0961±0.2789 | 13.6 (8) | 0.5717 (0.4760~0.6673) | 0.1347 | 0.0685±0.0398 | 93.7 (6) |
| BCG_2330 | 0.0547±0.2815 | 6.8 (4) | 0.6705 (0.5870~0.7539) | 0.0004 | 0.0117±0.0158 | 95.8 (4) |
| BCG_2765 | 0.0592±0.0393 | 3.4 (2) | 0.5395 (0.4470~0.6319) | 0.4102 | 0.0565±0.0458 | 97.9 (2) |
| BCG_3488c | 0.0629±0.2816 | 3.4 (2) | 0.5200 (0.4286~0.6113) | 0.6771 | 0.0285±0.0302 | 91.7 (8) |
| Ag85 | 0.0562±0.2816 | 6.8 (4) | 0.5640 (0.4733~0.6547) | 0.1816 | 0.0175±0.0208 | 94.8 (5) |
| HspX | 0.0201±0.0165 | 3.4 (2) | 0.5200 (0.4257~0.6144) | 0.6758 | 0.0223±0.0186 | 95.8 (4) |
| CysA | 0.006±0.0325 | 1.7 (1) | 0.5560 (0.4610~0.6509) | 0.2445 | 0.0126±0.0392 | 95.8 (4) |
| Rv1605 | 0.0817±0.2792 | 1.7 (1) | 0.5514 (0.4574~0.6453) | 0.2836 | 0.0532±0.036 | 96.9 (3) |
| Rv3593 | 0.04±0.0299 | 5.1 (3) | 0.6312 (0.5369~0.7255) | 0.0062 | 0.0511±0.0332 | 95.8 (4) |
| MAV5183 | 0.12±0.1248 | 1.7 (1) | 0.5672 (0.4735~0.6608) | 0.1627 | 0.1755±0.229 | 95.8 (4) |
| MAV4300 | 0.1026±0.2809 | 3.4 (2) | 0.5245 (0.4279~0.6210) | 0.6098 | 0.0779±0.0695 | 96.9 (3) |
| LAM | 0.2026±0.3073 | 49.2 (29) | 0.8397 (0.7694~0.9100) | <0.0001 | 0.0372±0.0339 | 97.9 (2) |
Table 7.
Effect of antigen combination to increase the sensitivity




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download