Abstract
Human cytomegalovirus (CMV) continues to be a major threat against solid-organ transplant recipients despite significant advancements in its prophylaxis and therapy. Primary CMV infection or reactivation of latent CMV in the transplant recipients may cause CMV diseases such as flu-like viral syndrome and tissue-invasive CMV disease. In addition, CMV infection in the recipients is associated with graft rejection and higher risk of other opportunistic infections, which are collectively known as the “indirect effects” of CMV infection. Prevention strategies with antiviral drugs including ganciclovir remarkably decreased CMV disease and the “indirect effects”. Two commonly employed strategies are universal prophylaxis and preemptive therapy. However, gangciclovir-resistant CMV has emerged due to mutations in CMV UL97 and UL54 genes, now requiring alternative therapeutic options to be developed. This review provides an overview of CMV infection and disease, “indirect effects” on hosts, prevention strategies, and drug resistance in solid-organ transplant recipients.
Go to : 
REFERENCES
1). Davison AJ, Dolan A, Akter P, Addison C, Dargan DJ, Alcendor DJ, et al. The human cytomegalovirus genome revisited: comparison with the chimpanzee cytomegalovirus genome. J Gen Virol. 2003; 84:17–28.

2). Söderberg-Nauclér C. Does cytomegalovirus play a causative role in the development of various inflammatory diseases and cancer? J Intern Med. 2006; 259:219–46.

3). Kotton CN, Kumar D, Caliendo AM, Asberg A, Chou S, Danziger-Isakov L, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013; 96:333–60.

4). Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002; 34:1094–7.

5). Razonable RR. Cytomegalovirus infection after liver transplantation: current concepts and challenges. World J Gastroenterol. 2008; 14:4849–60.

6). Patel R, Paya CV. Infections in solid-organ transplant recipients. Clin Microbiol Rev. 1997; 10:86–124.

7). Portela D, Patel R, Larson-Keller JJ, Ilstrup DM, Wiesner RH, Steers JL, et al. OKT3 treatment for allograft rejection is a risk factor for cytomegalovirus disease in liver transplantation. J Infect Dis. 1995; 171:1014–8.

8). Hassan-Walker AF, Kidd IM, Sabin C, Sweny P, Griffiths PD, Emery VC. Quantity of human cytomegalovirus (CMV) DNAemia as a risk factor for CMV disease in renal allograft recipients: relationship with donor/recipient CMV serostatus, receipt of augmented methylprednisolone and antithymocyte globulin (ATG). J Med Virol. 1999; 58:182–7.

9). Streblow DN, Orloff SL, Nelson JA. Acceleration of allograft failure by cytomegalovirus. Curr Opin Immunol. 2007; 19:577–82.

10). Kalil AC, Levitsky J, Lyden E, Stoner J, Freifeld AG. Meta-analysis: the efficacy of strategies to prevent organ disease by cytomegalovirus in solid organ transplant recipients. Ann Intern Med. 2005; 143:870–80.

11). Sedmak DD, Knight DA, Vook NC, Waldman JW. Divergent patterns of ELAM-1, ICAM-1, and VCAM-1 expression on cytomegalovirus-infected endothelial cells. Transplantation. 1994; 58:1379–85.
12). Craigen JL, Yong KL, Jordan NJ, MacCormac LP, Westwick J, Akbar AN, et al. Human cytomegalovirus infection up-regulates interleukin-8 gene expression and stimulates neutrophil transendothelial migration. Immunology. 1997; 92:138–45.

13). Burns LJ, Pooley JC, Walsh DJ, Vercellotti GM, Weber ML, Kovacs A. Intercellular adhesion molecule-1 expression in endothelial cells is activated by cytomegalovirus immediate early proteins. Transplantation. 1999; 67:137–44.
14). Kim MS, Yi HA, Lee CH. Human Cytomegalovirus Induces Intercellular Adhesion Molecule-1 Expression in a Monocytic Cell Line, THP-1. J Bacteriol Virol. 2008; 38:39–46.

15). Zhou YF, Yu ZX, Wanishsawad C, Shou M, Epstein SE. The immediate early gene products of human cytomegalovirus increase vascular smooth muscle cell migration, proliferation, and expression of PDGF beta-receptor. Biochem Biophys Res Commun. 1999; 256:608–13.
16). Squizzato A, Gerdes VE, Büller HR. Effects of human cytomegalovirus infection on the coagulation system. Thromb Haemost. 2005; 93:403–10.

17). Womer KL, Vella JP, Sayegh MH. Chronic allograft dysfunction: mechanisms and new approaches to therapy. Semin Nephrol. 2000; 20:126–47.
18). Thomas LD, Milstone AP, Miller GG, Loyd JE, Stephen Dummer J. Long-term outcomes of cytomegalovirus infection and disease after lung or heart-lung transplantation with a delayed ganciclovir regimen. Clin Transplant. 2009; 23:476–83.

19). Madalosso C, de Souza NF Jr, Ilstrup DM, Wiesner RH, Krom RA. Cytomegalovirus and its association with hepatic artery thrombosis after liver transplantation. Transplantation. 1998; 66:294–7.
20). Gao LH, Zheng SS. Cytomegalovirus and chronic allograft rejection in liver transplantation. World J Gastroenterol. 2004; 10:1857–61.

21). Potena L, Valantine HA. Cytomegalovirus-associated allograft rejection in heart transplant patients. Curr Opin Infect Dis. 2007; 20:425–31.

22). Snydman DR. The case for cytomegalovirus prophylaxis in solid organ transplantation. Rev Med Virol. 2006; 16:289–95.

23). Fishman JA, Emery V, Freeman R, Pascual M, Rostaing L, Schlitt HJ, et al. Cytomegalovirus in transplantation - challenging the status quo. Clin Transplant. 2007; 21:149–58.

24). Freeman RB Jr. The ‘indirect’ effects of cytomegalovirus infection. Am J Transplant. 2009; 9:2453–8.
25). George MJ, Snydman DR, Werner BG, Griffith J, Falagas ME, Dougherty NN, et al. The independent role of cytomegalovirus as a risk factor for invasive fungal disease in orthotopic liver transplant recipients. Boston Center for Liver Transplantation CMVIG-Study Group. Cytogam, MedImmune, Inc. Gaithersburg, Maryland. Am J Med. 1997; 103:106–13.
26). Muñoz P, Rodríguez C, Bouza E, Palomo J, Yañez JF, Domínguez MJ, et al. Risk factors of invasive aspergillosis after heart transplantation: protective role of oral itraconazole prophylaxis. Am J Transplant. 2004; 4:636–43.

27). Husni RN, Gordon SM, Longworth DL, Arroliga A, Stillwell PC, Avery RK, et al. Cytomegalovirus infection is a risk factor for invasive aspergillosis in lung transplant recipients. Clin Infect Dis. 1998; 26:753–5.

28). Peleg AY, Husain S, Qureshi ZA, Silveira FP, Sarumi M, Shutt KA, et al. Risk factors, clinical characteristics, and outcome of Nocardia infection in organ transplant recipients: a matched case-control study. Clin Infect Dis. 2007; 44:1307–14.

29). Mañez R, Breinig MC, Linden P, Wilson J, Torre-Cisneros J, Kusne S, et al. Posttransplant lymphoproliferative disease in primary Epstein-Barr virus infection after liver transplantation: the role of cytomegalovirus disease. J Infect Dis. 1997; 176:1462–7.

30). Mendez JC, Dockrell DH, Espy MJ, Smith TF, Wilson JA, Harmsen WS, et al. Human beta-herpesvirus interactions in solid organ transplant recipients. J Infect Dis. 2001; 183:179–84.
31). Razonable RR, Burak KW, van Cruijsen H, Brown RA, Charlton MR, Smith TF, et al. The pathogenesis of hepatitis C virus is influenced by cytomegalovirus. Clin Infect Dis. 2002; 35:974–81.

32). Ramanan P, Razonable RR. Cytomegalovirus infections in solid organ transplantation: a review. Infect Chemother. 2013; 45:260–71.

33). Arthurs SK, Eid AJ, Pedersen RA, Kremers WK, Cosio FG, Patel R, et al. Delayed-onset primary cytomegalovirus disease and the risk of allograft failure and mortality after kidney transplantation. Clin Infect Dis. 2008; 46:840–6.

34). Humar A, Lebranchu Y, Vincenti F, Blumberg EA, Punch JD, Limaye AP, et al. The efficacy and safety of 200 days valganciclovir cytomegalovirus prophylaxis in high-risk kidney transplant recipients. Am J Transplant. 2010; 10:1228–37.

35). Palmer SM, Limaye AP, Banks M, Gallup D, Chapman J, Lawrence EC, et al. Extended valganciclovir prophylaxis to prevent cytomegalovirus after lung transplantation: a randomized, controlled trial. Ann Intern Med. 2010; 152:761–9.
36). Abate D, Saldan A, Fiscon M, Cofano S, Paciolla A, Furian L, et al. Evaluation of cytomegalovirus (CMV)-specific T cell immune reconstitution revealed that baseline antiviral immunity, prophylaxis, or preemptive therapy but not antithymocyte globulin treatment contribute to CMV-specific T cell reconstitution in kidney transplant recipients. J Infect Dis. 2010; 202:585–94.

37). Razonable RR. Strategies for managing cytomegalovirus in transplant recipients. Expert Opin Pharmacother. 2010; 11:1983–97.

38). Manuel O, Kralidis G, Mueller NJ, Hirsch HH, Garzoni C, van Delden C, et al. Impact of antiviral preventive strategies on the incidence and outcomes of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2013; 13:2402–10.

39). Myhre HA, Haug Dorenberg D, Kristiansen KI, Rollag H, Leivestad T, Asberg A, et al. Incidence and outcomes of ganciclovir-resistant cytomegalovirus infections in 1244 kidney transplant recipients. Transplantation. 2011; 92:217–23.

40). Boivin G, Goyette N, Farhan M, Ives J, Elston R. Incidence of cytomegalovirus UL97 and UL54 amino acid substitutions detected after 100 or 200 days of valganciclovir prophylaxis. J Clin Virol. 2012; 53:208–13.

41). Li F, Kenyon KW, Kirby KA, Fishbein DP, Boeckh M, Limaye AP. Incidence and clinical features of ganciclovir-resistant cytomegalovirus disease in heart transplant recipients. Clin Infect Dis. 2007; 45:439–47.
42). Lurain NS, Chou S. Antiviral drug resistance of human cytomegalovirus. Clin Microbiol Rev. 2010; 23:689–712.

44). Cvetković RS, Wellington K. Valganciclovir: a review of its use in the management of CMV infection and disease in immunocompromised patients. Drugs. 2005; 65:859–78.
45). Chou S. Cytomegalovirus UL97 mutations in the era of ganciclovir and maribavir. Rev Med Virol. 2008; 18:233–46.

46). Baek MC. Screening of Peptide Libraries to Investigate the Substrate Specificity of UL97 Protein Kinase from Human Cytomegalovirus. J Bacteriol Virol. 2006; 36:119–24.

47). Le Page AK, Jager MM, Iwasenko JM, Scott GM, Alain S, Rawlinson WD. Clinical aspects of cytomegalovirus antiviral resistance in solid organ transplant recipients. Clin Infect Dis. 2013; 56:1018–29.

48). Jacobson MA, Drew WL, Feinberg J, O'Donnell JJ, Whitmore PV, Miner RD, et al. Foscarnet therapy for ganciclovir-resistant cytomegalovirus retinitis in patients with AIDS. J Infect Dis. 1991; 163:1348–51.

Go to : 
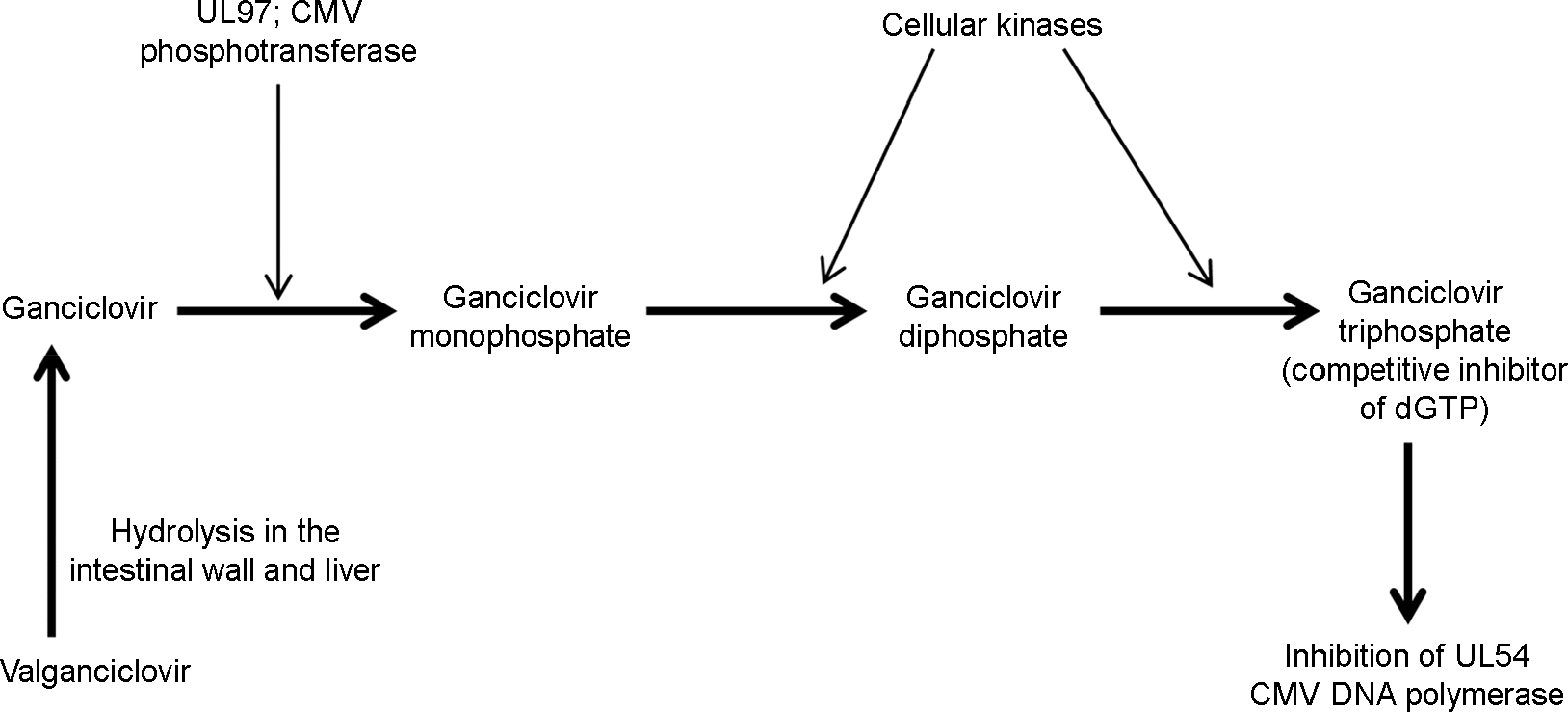 | Figure 1.Ganciclovir metabolism and mode of action. Ganciclovir requires three consecutive phosphorylation steps for its antiviral activity. The first phosphorylation step is carried out by a viral phosphotransferase encoded by CMV UL97 gene. Cellular kinases catalyze two additional rounds of phosphorylation. Ganciclovir triphosphate is a competitive inhibitor of dGTP (deoxyguanosine triphosphate), and preferentially inhibits viral DNA polymerases encoded by CMV UL54 gene. Valganciclovir is a prodrug of ganciclovir. |
Table 1.
Definitions of cytomegalovirus (CMV) infection and disease
Table 2.
Universal prophylaxis versus preemptive therapy




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download