Abstract
Marine algae are rich sources of various biologically active compounds with potential pharmaceutical properties. In the present study, we investigated the inhibitory effects of Plocamium telfairiae extract (PTE) on proinflammatory cytokine production in bone marrow-derived macrophage (BMDMs) and dendritic cells (BMDCs). PTE pre-treatment in LPS-stimulated BMDMs and BMDCs showed a strong inhibition on interleukin (IL)-12 p40, IL-6, and tumor necrosis factor (TNF)-α production as compared to non-treated controls. PTE pre-treatment showed significant inhibition on phosphorylation of mitogen-activated protein kinases and degradation of inhibitor of kappa B (IκBα). Taken together, these results suggest that PTE may have potential anti-inflammatory property and hence, warrant further studies concerning the potentials of PTE for medicinal purpose.
Go to : 
REFERENCES
2). Barton GM. A calculated response: control of inflammation by the innate immune system. J Clin Invest. 2008; 118:413–20.

3). Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006; 124:783–801.

5). Mogensen TH. Pathogen recognition and inflammatory signalling in innate immune defenses. Clin Microbiol Rev. 2009; 22:240–73.
7). Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001; 13:85–94.

8). Thalhamer T, McGrath MA, Harnett MM. MAPKs and their relevance to arthritis and inflammation. Rheumatology. 2008; 47:409–14.

9). Manzoor Z, Koh YS. Mitogen-activated protein kinases in inflammation. J Bacteriol Virol. 2012; 42:189–95.

10). Weiss T, Shalit I, Blau H, Werber S, Halperin D, Levitov A, et al. Anti-inflammatory effects of moxifloxacin on activated human monocytic cells: inhibition of NF-kappaB and mitogen-activated protein kinase activation and of synthesis of proinflammatory cytokines. Antimicrob Agents Chemother. 2004; 48:1974–82.
11). O'Sullivan AW, Wang JH, Redmond HP. NF-kappaB and p38 MAPK inhibition improve survival in endotoxin shock and in a cecal ligation and puncture model sepsis in combination with antibiotic therapy. J Surg Res. 2009; 152:46–53.
12). Shaked G, Czeiger D, Dukhno O, Levy I, Artru AA, Shapira Y, et al. Ketamine improves survival and suppresses IL-6 and TNF alpha production in a model of gram-negative bacterial sepsis in rats. Resuscitation. 2004; 62:237–42.
13). Blunt JW, Copp BR, Hu WP, Munro MH, Northcote PT, Prinsep MR. Marine natural products. Nat Prod Rep. 2009; 26:170–244.

14). Kim JY, Yoon MY, Cha MR, Hwang JH, Park E, Choi SU, et al. Methanolic extracts of Plocamium telfairiae induce cytotoxicity and caspase-dependent apoptosis in HT-29 human colon carcinoma cells. J Med Food. 2007; 10:587–93.
15). Huang HL, Wang BG. Antioxidant capacity and lipophilic content of seaweeds collected from the Qingdao coastline. J Agric Food Chem. 2004; 52:4993–7.

16). Koo JE, Hong HJ, Dearth A, Kobayashi KS, Koh YS. Intracellular invasion of Orientia tsutsugamushi activates inflammasome in ASC-dependent manner. PLoS One. 2012; 7:e39042.
17). Chae D, Manzoor Z, Kim SC, Kim S, Oh TH, Yoo ES, et al. Apo-9′-fucoxanthinone, isolated from Sargassum muticum, inhibits CpG-induced inflammatory response by attenuating the mitogen-activated protein kinase pathway. Mar Drugs. 2013; 11:3272–87.
18). Manzoor Z, Kim S, Chae D, Yoo ES, Kang HK, Hyun JW, et al. Sea lettuce (Ulva fasciata) extract has an inhibitory effect on pro-inflammatory cytokine production in CpG-stimulated bone marrow-derived macrophages and dendritic cells. Food Sci Biotechnol. 2013; 22:781–6.
19). Manzoor Z, Mathema VB, Chae D, Kang HK, Yoo ES, Jeon YJ, et al. Octaphlorethol A inhibits the CpG-induced inflammatory response by attenuating the mitogen-activated protein kinase and NF-κB pathways. Biosci Biotechnol Biochem. 2013; 77:1970–2.

20). Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010; 11:373–84.

21). Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003; 3:133–46.

22). Adorini L. Interleukin-12, a key cytokine in Th1-mediated autoimmune diseases. Cell Mol Life Sci. 1999; 55:1610–25.

24). Mudter J, Neurath MF. IL-6 signaling in inflammatory bowel disease: pathophysiological role and clinical relevance. Inflamm Bowel Dis. 2007; 13:1016–23.

25). Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001; 11:372–7.

27). Rigato O, Ujvari S, Castelo A, Salomão R. Tumor necrosis factor alpha (TNF-alpha) and sepsis: evidence for a role in host defense. Infection. 1996; 24:314–8.
Go to : 
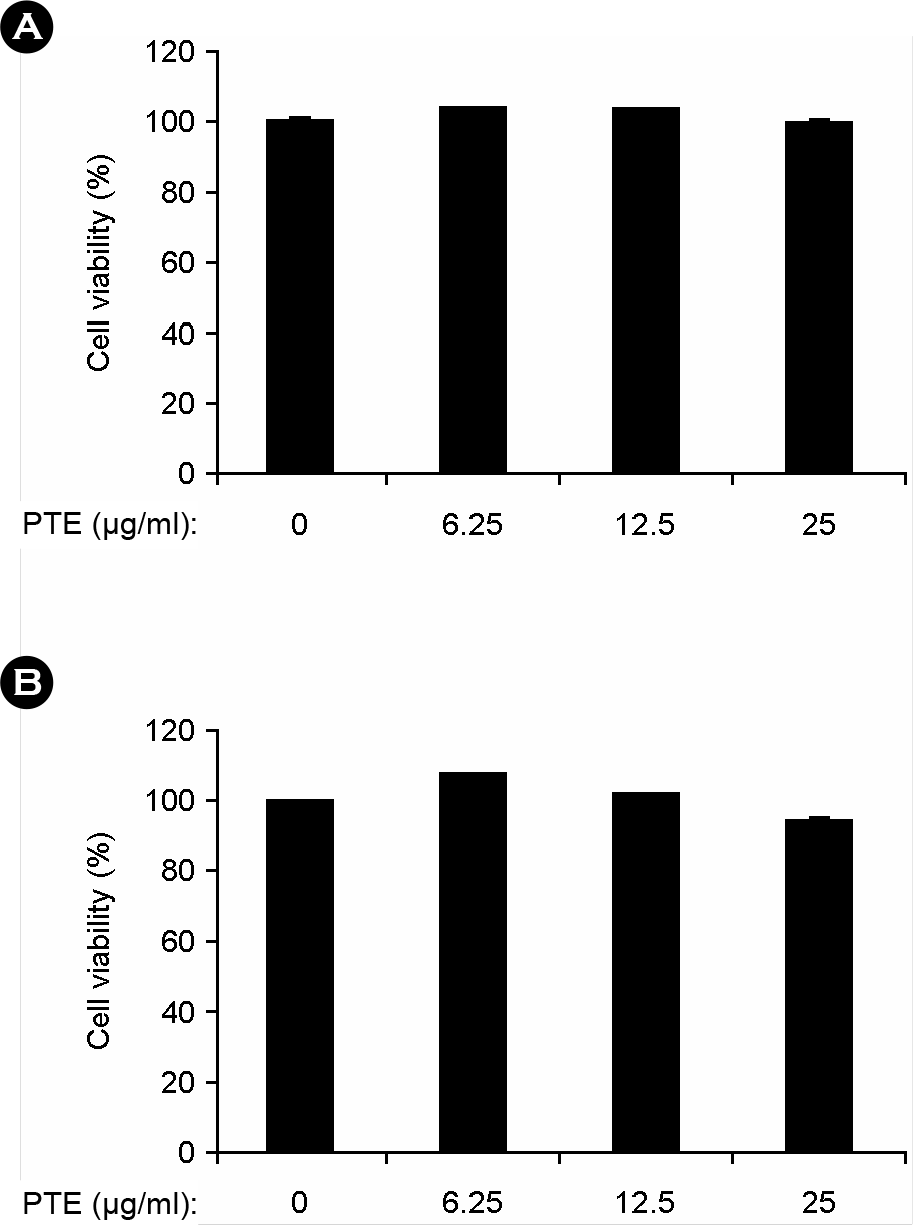 | Figure 1.Effects of PTE on cell viability in BMDMs and BMDCs. BMDMs (A) and BMDCs (B) were treated with the indicated concentrations of PTE for 1 h before stimulation with LPS (10 ng/ml). The cell viability was measured using MTT assay. Data are representative of three independent experiments. PTE, Plocamium telfairiae extract. |
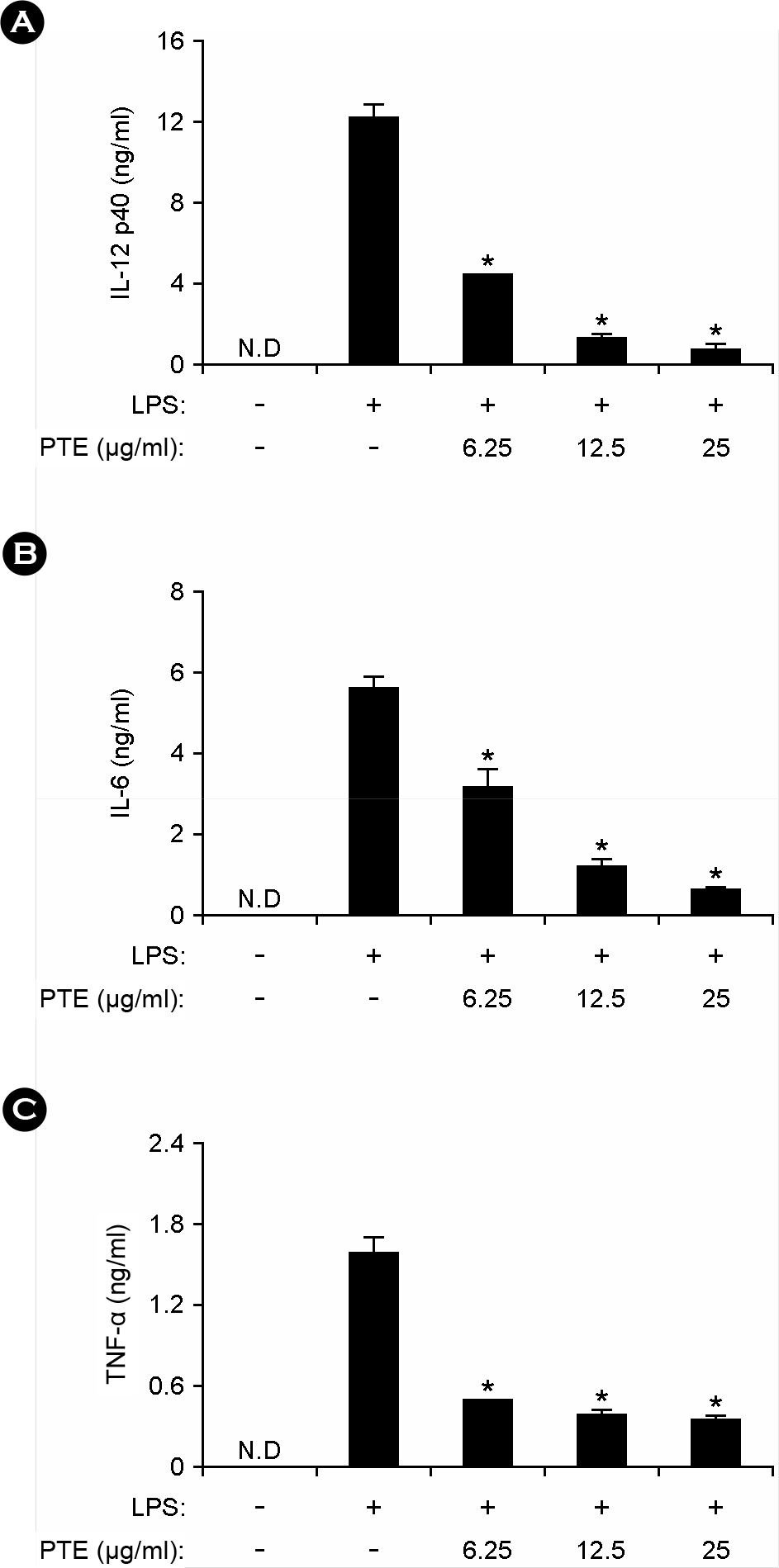 | Figure 2.Effects of PTE on IL-12 p40, IL-6, and TNF-α production in LPS-stimulated BMDMs. BMDMs were treated with PTE at the indicated doses for 1 h before stimulation with LPS (10 ng/ml). The concentrations of murine IL-12 p40 (A), IL-6 (B) and TNF-α (C) released into the culture medium were determined by ELISA. Data are representative of three independent experiments. PTE, Plocamium telfairiae extract; N.D., not detectable. *p < 0.05 versus PTE-untreated cells in the presence of LPS. |
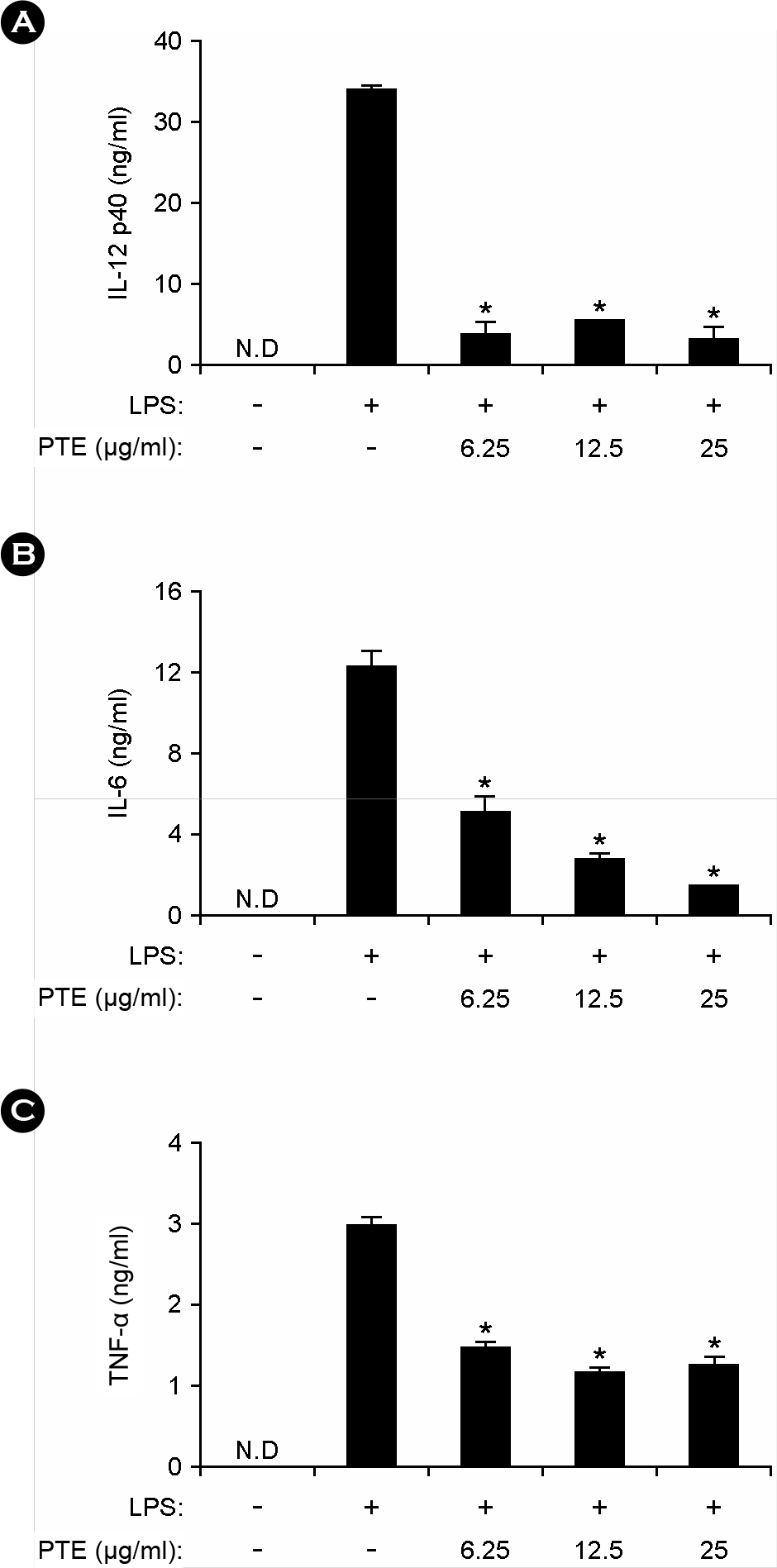 | Figure 3.Effects of PTE on IL-12 p40, IL-6 and TNF-α production in LPS-stimulated BMDCs. BMDCs were treated with PTE and the concentrations of murine IL-12 p40 (A), IL-6 (B) and TNF-α (C) measured as described in Fig. 2. Data are representative of three independent experiments. PTE, Plocamium telfairiae extract; N.D., not detectable. *p < 0.05 versus PTE-untreated cells in the presence of LPS. |
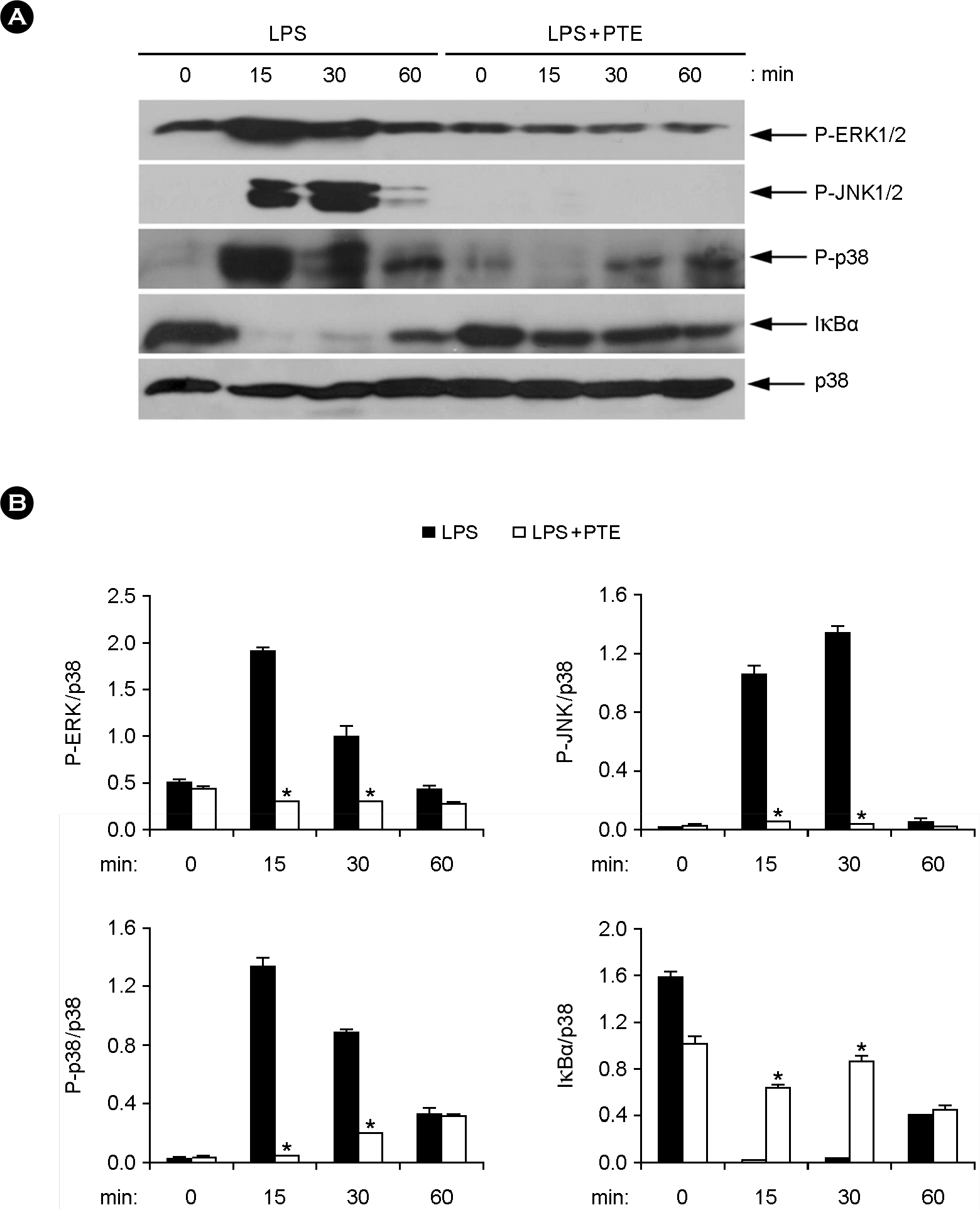 | Figure 4.Effects of PTE on the phosphorylation of MAPKs and degradation of IκBα in LPS-stimulated BMDMs. (A) BMDMs were pre-treated with or without PTE (25 μg/ml) for 1 h and then stimulated with LPS (10 ng/ml) for the indicated time periods. Cell lysates were analyzed by western blot analysis. Total p38 MAPK was taken as the loading control. Data are representative of three independent experiments. (B) Phosphorylation of ERK, JNK and p38 and expression of IκBα protein was quantified using scanning densitometry, and the band intensities were normalized by that of total p38 protein. PTE, Plocamium telfairiae extract. *p < 0.05 versus PTE-untreated cells in the presence of LPS. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download