Abstract
Typhoid fever, a serious systemic infection caused by Salmonella enterica serovar Typhi, breaks out in developing countries. However, existing vaccines only induce relatively low protective effects with humoral responses and do not stimulate secondary immune response, especially to young people. The objective of this study is to evaluate the immunogenicity of the vaccine containing virulence capsular polysaccharide (Vi) conjugated with the optimal ratios of non-toxic variant of diphtheria toxin (CRM197) in mice. Six-week-old BALB/c female mice were injected intraperitoneally three times at intervals of 14 days and sera were collected on days 0, 14, 28, 42 and 56 post-injection. The efficacy of the vaccine was evaluated by comparing between negative control group injected with PBS and vaccine groups injected with Vi or Vi-CRM197 conjugate of different ratio. Vi and CRM197-specific antibody responses were evaluated using enzyme-linked immunosorbent assay. The result showed that Vi-CRM197-1 group revealed the highest and significant Vi-specific IgG immune responses among the other groups and Vi group (p < 0.01). In conclusion, Vi-CRM197-1 conjugate vaccine induced the highest humoral immune response in mice and may be used as an effective vaccine to replace the existing typhoid vaccine for infants under 2 years old.
Go to : 
REFERENCES
1). Cui C, Carbis R, An SJ, Jang H, Czerkinsky C, Szu SC, et al. Physical and chemical characterization and immunologic properties of Salmonella enterica serovar typhi capsular polysaccharide-diphtheria toxoid conjugates. Clin Vaccine Immunol. 2010; 17:73–9.
2). Toobak H, Rasooli I, Talei D, Jahangiri A, Owlia P, Darvish Alipour Astaneh S. Immune response variations to Salmonella enterica serovar Typhi recombinant porin proteins in mice. Biologicals. 2013; 41:224–30.
3). Lu YJ, Zhang F, Sayeed S, Thompson CM, Szu S, Anderson PW, et al. A bivalent vaccine to protect against Streptococcus pneumoniae and Salmonella typhi. Vaccine. 2012; 30:3405–12.
4). No authors listed. Typhoid vaccines: WHO position paper. Wkly Epidemiol Rec. 2008; 83:49–59.
5). Marathe SA, Lahiri A, Negi VD, Chakravortty D. Typhoid fever & vaccine development: a partially answered question. Indian J Med Res. 2012; 135:161–9.
6). An SJ, Yoon YK, Kothari S, Kim DR, Kim JA, Kothari N, et al. Immune suppression induced by Vi capsular polysaccharide is overcome by Vi-DT conjugate vaccine. Vaccine. 2012; 30:1023–8.

7). Guzman CA, Borsutzky S, Griot-Wenk M, Metcalfe IC, Pearman J, Collioud A, et al. Vaccines against typhoid fever. Vaccine. 2006; 24:3804–11.

8). DeRoeck D, Ochiai RL, Yang J, Anh DD, Alag V, Clemens JD. Typhoid vaccination: the Asian experience. Expert Rev Vaccines. 2008; 7:547–60.

9). An SJ, Yoon YK, Kothari S, Kothari N, Kim JA, Lee E, et al. Physico-chemical properties of Salmonella typhi Vi polysaccharide-diphtheria toxoid conjugate vaccines affect immunogenicity. Vaccine. 2011; 29:7618–23.
10). Jones C. Vaccines based on the cell surface carbohydrates of pathogenic bacteria. An Acad Bras Cienc. 2005; 77:293–324.

11). Croxtall JD, Keating GM. Pneumococcal polysaccharide protein D-conjugate vaccine (Synflorix; PHiD-CV). Paediatr Drugs. 2009; 11:349–57.

12). Micoli F, Rondini S, Pisoni I, Proietti D, Berti F, Costantino P, et al. Vi-CRM197 as a new conjugate vaccine against Salmonella Typhi. Vaccine. 2011; 29:712–20.
13). Snape MD, Perrett KP, Ford KJ, John TM, Pace D, Yu LM, et al. Immunogenicity of a tetravalent meningococcal glycoconjugate vaccine in infants: a randomized controlled trial. JAMA. 2008; 299:173–84.

14). Jackson LA, Jacobson RM, Reisinger KS, Anemona A, Danzig LE, Dull PM. A randomized trial to determine the tolerability and immunogenicity of a quadrivalent meningococcal glycoconjugate vaccine in healthy adolescents. Pediatr Infect Dis J. 2009; 28:86–91.

15). Shinefield HR. Overview of the development and current use of CRM197 conjugate vaccines for pediatric use. Vaccine. 2010; 28:4335–9.
16). Rondini S, Micoli F, Lanzilao L, Pisoni I, Di Cioccio V, Saul AJ, et al. Characterization of Citrobacter sp. line 328 as a source of Vi for a Vi-CRM197 glycoconjugate vaccine against Salmonella Typhi. J Infect Dev Ctries. 2012; 6:763–73.
17). Jang H, Yoon YK, Kim JA, Kim HS, An SJ, Seo JH, et al. Optimization of Vi capsular polysaccharide production during growth of Salmonella enterica serotype Typhi Ty2 in a bioreactor. J Biotechnol. 2008; 135:71–7.
18). Santander J, Roland KL, Curtiss R 3rd. Regulation of Vi capsular polysaccharide synthesis in Salmonella enterica serotype Typhi. J Infect Dev Ctries. 2008; 2:412–20.

19). Fiorino F, Ciabattini A, Rondini S, Pozzi G, Martin LB, Medaglini D. Immunization with the conjugate vaccine Vi-CRM197 against Salmonella Typhi Induces Vi-specific mucosal and systemic immune responses in mice. Vaccine. 2012; 30:6111–4.
22). van Damme P, Kafeja F, Anemona A, Basile V, Hilbert AK, De Coster I, et al. Safety, immunogenicity and dose ranging of a new Vi-CRM197 conjugate vaccine against typhoid fever: randomized clinical testing in healthy adults. PLoS One. 2011; 6:e25398.
Go to : 
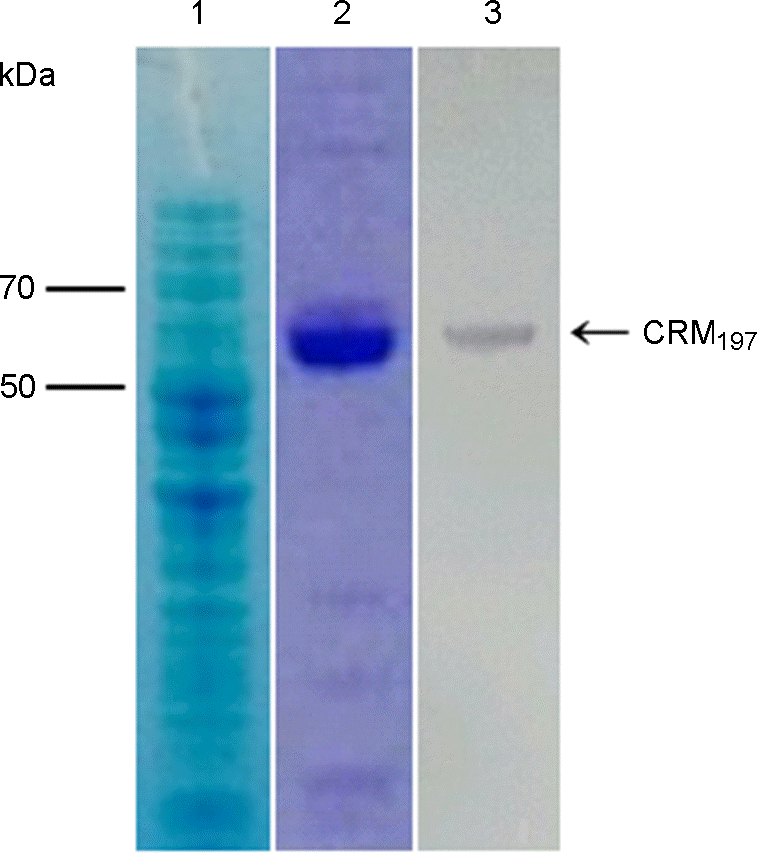 | Figure 1.
SDS-PAGE and Western blot of recombinant CRM197 protein induced by 0.02% arabinose in E. coli. The total extracts (lane 1) of E. coli containing pBAD-CRM197 and purified CRM197 (lane 2) were separated by SDS-PAGE and stained with Coomassie blue, analyzed by Western blot of the duplicated gel of lane 1 using anti-His antibody and anti-mouse IgG AP conjugated antibody (lane 3). |
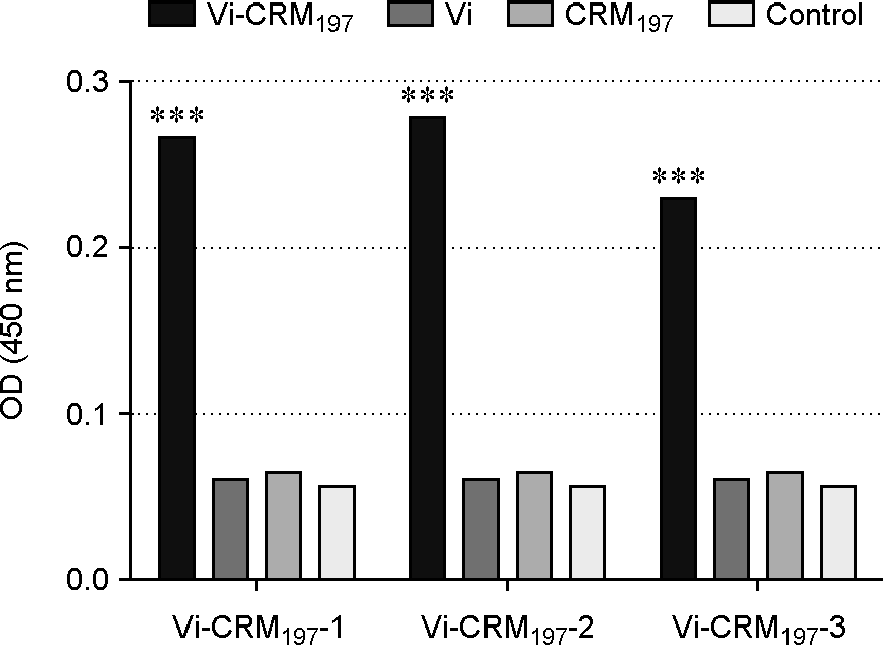 | Figure 2.
Analysis of Vi-CRM197 conjugate levels by sandwich ELISA. For sandwich ELISA, plates were coated with anti-His antibody. Vi, CRM197 and Vi-CRM197 were added to each well. Anti-Vi polyclonal antibody was added and then anti-mouse IgG AP-conjugated antibody was added to each well. ***, Significant difference in Vi-CRM197 conjugate compared with the control group (p < 0.001). |
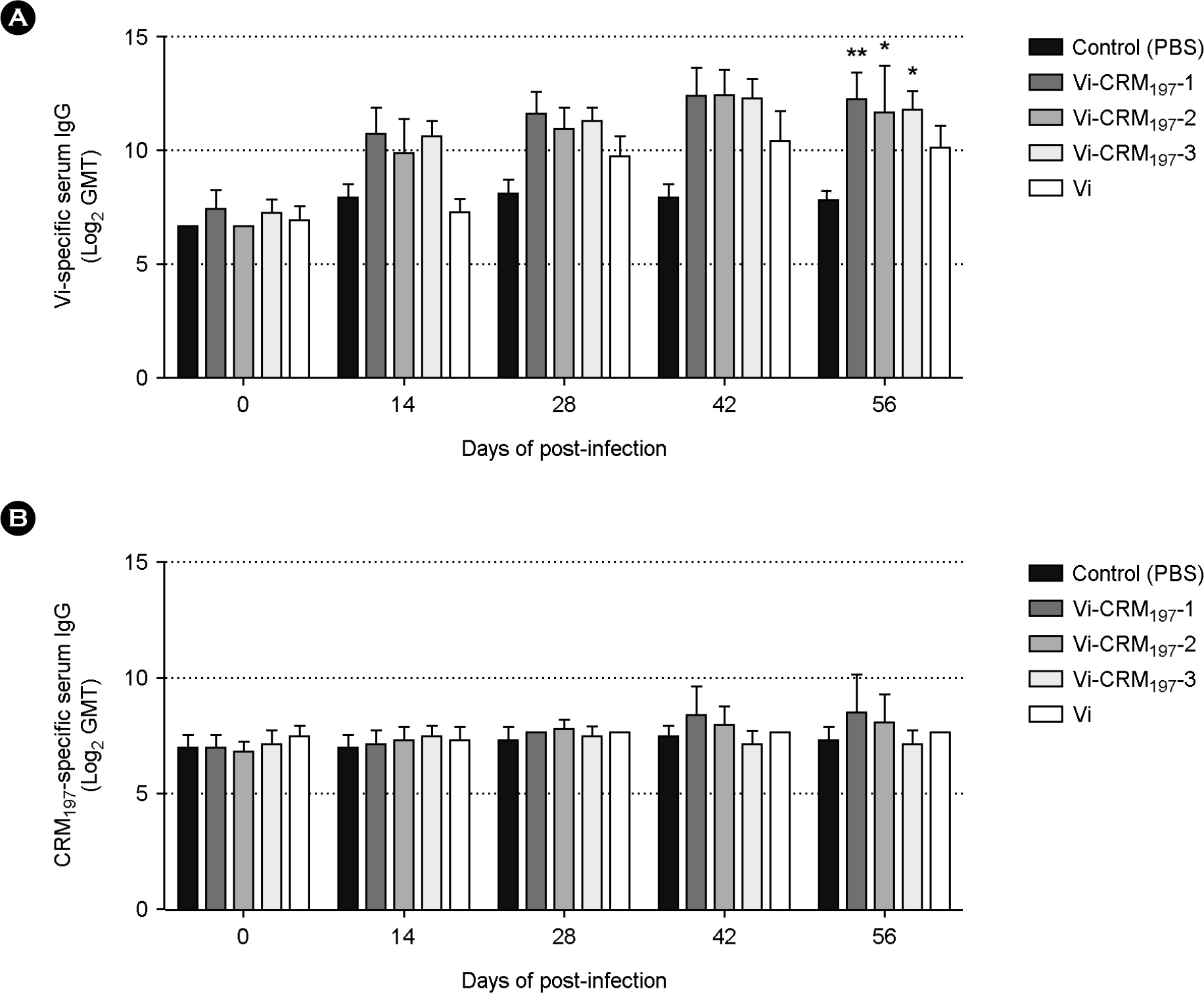 | Figure 3.
Vi and CRM197-specific serum IgG responses in mice following dosing with Vi and Vi-CRM197 conjugates. BALB/c mice were immunized intraperitoneally three times at 14-day internals with 2.5 μg of Vi-CRM197. Values are reported as log2 geometric mean titer (GMT) and error bars indicate 95% confidence intervals. **p < 0.01 and *p < 0.05 versus control (PBS) immunized mice. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download