Abstract
According to a United States study, 13 cases of vancomycin-resistant Staphylococcus aureus (VRSA) have been reported to date. In 2001, a survey conducted in Korea revealed that 0.5% of methicillin-resistant S. aureus (MRSA) isolates have a vancomycin minimum inhibitory concentration (MIC) of 4 μg/ml, and are thus referred to as vancomycin intermediate S. aureus (VISA). However there are no reports of VISA found in primary hospitals. We evaluated the MIC of vancomycin in MRSA samples obtained from primary hospitals to determine whether VISA was present in primary hospitals. The population analysis was performed to determine whether hetero-VISA was present in primary hospitals. As a result, twenty of the 103 isolates were S. aureus which were all MRSA and the vancomycin MIC was similar to that seen in tertiary hospitals. Population analysis confirmed that three strains were hetero-VISA, by showing that one strain grew in 8 μg/ml vancomycin and that two strains grew in 4 μg/ml vancomycin. In conclusion, hetero-VISA was detected in Korean primary hospitals, which may develop into VISA, however a larger sample size will be needed to confirm these results.
Go to : 
REFERENCES
1). Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover FC, et al. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin suscetibility. J Antimicrob Chemother. 1997; 40:135–6.
2). Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; Sixteenth Informational Supplement, M100-S16. Wayne, PA: Clinical and Laboratory Standards Institute;2006.
3). Hiramatsu K, Aritaka N, Hanaki H, Kawasaki S, Hosoda Y, Hori S, et al. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogenously resistant to vancomycin. Lancet. 1997; 350:1670–3.
4). Hafer C, Lin Y, Kornblum J, Lowy FD, Uhlemann AC. Contribution of selected gene mutations to resistance in clinical isolates of vancomycin-intermediate Staphylococcus aureus. Antimicrob Agents Chemother. 2012; 56:5845–51.
5). Kim HB, Lee YS, Kim BS, Cha JO, Kwon SU, Lee HJ, et al. Prevalence and clinical implications of Staphylococcus aureus with a vancomycin MIC of 4 microg/ml in Korea. Microb Drug Resist. 2006; 12:33–8.
6). Henry D. Isenberg. Clinical Microbiology Procedures Handbook. 2nd ed.American Society for Microbiology;2004.
7). Kim MN, Pai CH, Woo JH, Ryu JS, Hiramatsu K. Vancomycin-intermediate Staphylococcus aureus in Korea. J Clin Microbiol. 2000; 38:3879–81.
8). Hong KH, Park JS, Kim EC. Two Cases of Vancomycin-intermediate Staphylococcus aureus Isolated from Joint Tissue or Wound. Korean J Lab Med. 2008; 28:444–8.
9). Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med. 2006; 166:2138–44.
10). Cui L, Isii T, Fukuda M, Ochiai T, Neoh HM, Camargo IL, et al. An RpoB mutation confers dual heteroresistance to daptomycin and vancomycin in Staphylococcus aureus. Antimicrob Agents Chemother. 2010; 54:5222–33.
11). Passalacqua KD, Satola SW, Crispell EK, Read TD. A mutation in the PP2C phosphatase gene in a Staphylococcus aureus US300 clinical isolate with reduced susceptibility to vancomycin and daptomycin. Antimicrob Agents Chemother. 2012; 56:5212–23.
12). Han JH, Edelstein PH, Lautenbach E. Reduced vancomycin susceptibility and staphylococcal cassette chromosome mec(SCCmec) type distribution in methicillin-resistant Staphylococcu aureus bacteraemia. J Antimicrob Chemother. 2012; 67:2346–9.
13). Leonard SN. Synergy between vancomycin and nafcillin against Staphylococcus aureus in an in vitro pharmacokinetic/pharmacodynamic model. PLoS One. 2012; 7:e42103.
14). Song JS, Choe PG, Song KH, Cho JH, Kim SH, Bang JH, et al. Multicenter study for frequency and clinical features of community-associated methicillin-resistant Staphylococcus aureus in Korea. Infect Chemother. 2006; 38:325–33.
15). Park GI, Lee GS, Shin JW, Lee KW, Chong YS. The detection of hetero-vancomycin intermediate MRSA with Mu-3 agar. Korean J Clin Microbiol. 1999; 2:S52.
Go to : 
 | Figure 1.
The count of CFU at each vancomycin concentration. After 10-fold dilution of bacterial solution (strain 0501) from 10−1 to 10−7, 100 μl inoculated to each serial dilution of vancomycin BHIA with sterile tip, applied evenly with a sterile swab and incubated at 35°C for 24 hours. We counted colony number and found the growth of 70 colonies of strain 0501 with 10−3 dilution at vancomycin 4 μg/ml. |
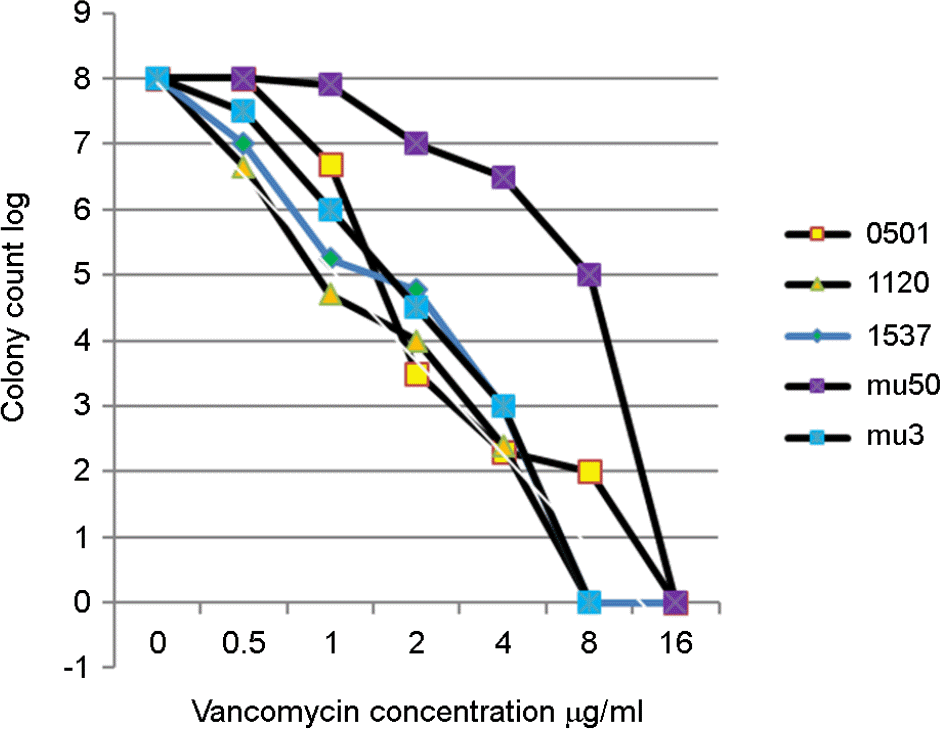 | Figure 2.
Logarithmically charted CFU at each vancomycin concentration. After 10-fold dilution of bacterial suspension from 10−1 to 10−7, 100 μl of each serial dilution was inoculated on BHIA containing vancomycin, After incubation the colony numbers were counted and the colony number at each vancomycin concentration was charted logarithmically. (axis X, vancomycin concentration range; 0∼16 μg/ml, axis Y, colony count at each dilution from 10−1 to 10−7, range; 0∼8) mu50, mu3; control strain, 0501,1120,1537; test strain |
Table 1.
Minimal inhibitory concentraion of MRSA isolates against vancomycin
| No | Specimen | MIC (μg/ml) | MIC 24 hr |
|---|---|---|---|
| 1 | 0501 | 2 | 4 |
| 2 | 1049 | 1 | 2 |
| 3 | 1054 | 1 | 2 |
| 4 | 1055 | 2 | 2 |
| 5 | 1056 | 1 | 1 |
| 6 | 1064 | 0.5 | 2 |
| 7 | 1120 | 1 | 4 |
| 8 | 1537 | 1 | 4 |
| 9 | 2041 | 1 | 2 |
| 10 | 2518 | 1 | 1 |
Table 2.
Growth rate of hetero-VISA strains at each vancomycin concentration
|
Colony count at each vancomycin concentration (μg/ml) |
||||||
|---|---|---|---|---|---|---|
| 0.25** | 0.5 | 1 | 2 | 4 | 8 | |
| 0501* | Confluent† | Confluent | 2.7 × 105 | 8.3 × 104 | 7.0 × 104 | 1.1 × 104 |
| 1120 | Confluent | 2.6 × 105 | 8.1 × 104 | 1.6 × 104 | 3.2 × 103 | 0 |
| 1537 | Confluent | Confluent | 1.6 × 105 | 5.5 × 104 | 3.0 × 103 | 0 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download