Abstract
Human Respiratory Syncytial virus (hRSV) is a leading cause of severe lower respiratory tract diseases in the pediatric population. RSV frequently causes severe morbidity and mortality in high risk groups including infants with congenital heart disease and the immunosuppressed patients. Although hRSV is recognized as a major public health threat and economic burden worldwide, there is no licensed vaccine and effective therapeutic agent. Viral nonstructural (NS) proteins have been known to play multiple functions for efficient viral replication and pathogenesis. Especially, diverse functions of influenza A virus NS1 have been extensively studies. Recent studies demonstrated that NS1 and NS2 of RSV also exert diverse functions to modulate cellular environment and antiviral immune responses. Since NS proteins of RSV are required for efficient replication and pathogenesis, NS mutant viruses have been tested as live-attenuated vaccines. This review will outline the recent progress in understanding the various functions of RSV NS1 and NS2.
Go to : 
REFERENCES
1). Hall CB1, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009; 360:588–98.

2). Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986; 140:543–6.

3). Pastey MK, Samal SK. Nucleotide sequence analysis of the non-structural NS1 (1C) and NS2 (1B) protein genes of bovine respiratory syncytial virus. J Gen Virol. 1995; 76:193–7.

4). Teng MN. The non-structural proteins of RSV: targeting interferon antagonists for vaccine development. Infect Disord Drug Targets. 2012; 12:129–37.
5). Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003; 289:179–86.

6). Nair H1, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010; 375:1545–55.

7). Shay DK1, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA. 1999; 282:1440–6.

8). Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med. 2000; 161:1501–7.

9). Varga SM, Braciale TJ. The adaptive immune response to respiratory syncytial virus. Curr Top Microbiol Immunol. 2013; 372:155–71.

10). Johnson JE, Gonzales RA, Olson SJ, Wright PF, Graham BS. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod Pathol. 2007; 20:108–19.

11). DeVincenzo JP, Wilkinson T, Vaishnaw A, Cehelsky J, Meyers R, Nochur S, et al. Viral load drives disease in humans experimentally infected with respiratory syncytial virus. Am J Respir Crit Care Med. 2010; 182:1305–14.

12). Hall CB, Walsh EE, Long CE, Schnabel KC. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis. 1991; 163:693–8.

13). Prince GA, Horswood RL, Camargo E, Koenig D, Chanock RM. Mechanisms of immunity to respiratory syncytial virus in cotton rats. Infect Immun. 1983; 42:81–7.

14). Prince GA, Horswood RL, Chanock RM. Quantitative aspects of passive immunity to respiratory syncytial virus infection in infant cotton rats. J Virol. 1985; 55:517–20.

15). Taylor G, Stott EJ, Bew M, Fernie BF, Cote PJ, Collins AP, et al. Monoclonal antibodies protect against respiratory syncytial virus infection in mice. Immunology. 1984; 52:137–42.
16). Kim TH, Lee HK. Innate immune recognition of respiratory syncytial virus infection. BMB Rep. 2014; 47:184–91.

17). Mukherjee S, Lukacs NW. Innate immune responses to respiratory syncytial virus infection. Curr Top Microbiol Immunol. 2013; 372:139–54.

18). Barik S. Respiratory syncytial virus mechanisms to interfere with type 1 interferons. Curr Top Microbiol Immunol. 2013; 372:173–91.

19). Spann KM, Tran KC, Collins PL. Effects of nonstructural proteins NS1 and NS2 of human respiratory syncytial virus on interferon regulatory factor 3, NF-kappaB, and proinflammatory cytokines. J Virol. 2005; 79:5353–62.
20). Swedan S, Andrews J, Majumdar T, Musiyenko A, Barik S. Multiple functional domains and complexes of the two non-structural proteins of human respiratory syncytial virus contribute to interferon suppression and cellular location. J Virol. 2011; 85:10090–100.

21). Atreya PL, Kulkarni S. Respiratory syncytial virus strain A2 is resistant to the antiviral effects of type I interferons and human MxA. Virology. 1999; 261:227–41.

22). Teng MN, Collins PL. Altered growth characteristics of recombinant respiratory syncytial viruses which do not produce NS2 protein. J Virol. 1999; 73:466–73.

23). Teng MN, Whitehead SS, Bermingham A, St Claire M, Elkins WR, Murphy BR, et al. Recombinant respiratory syncytial virus that does not express the NS1 or M2-2 protein is highly attenuated and immunogenic in chimpanzees. J Virol. 2000; 74:9317–21.

24). Spann KM, Tran KC, Chi B, Rabin RL, Collins PL. Suppression of the induction of alpha, beta, and lambda interferons by the NS1 and NS2 proteins of human respiratory syncytial virus in human epithelial cells and macrophages [corrected]. J Virol. 2004; 78:4363–9.
25). Okabayashi T, Kojima T, Masaki T, Yokota S, Imaizumi T, Tsutsumi H, et al. Type-III interferon, not type-I, is the predominant interferon induced by respiratory viruses in nasal epithelial cells. Virus Res. 2011; 160:360–6.

26). Ling Z1, Tran KC, Teng MN. Human respiratory syncytial virus nonstructural protein NS2 antagonizes the activation of beta interferon transcription by interacting with RIG-I. J Virol. 2009; 83:3734–42.

27). Swedan S, Musiyenko A, Barik S. Respiratory syncytial virus nonstructural proteins decrease levels of multiple members of the cellular interferon pathways. J Virol. 2009; 83:9682–93.

28). Xu X, Zheng J, Zheng K, Hou Y, Zhao F, Zhao D. Respiratory syncytial virus NS1 protein degrades STAT2 by inducing SOCS1 expression. Intervirology. 2014; 57:65–73.

29). Ramaswamy M, Shi L, Varga SM, Barik S, Behlke MA, Look DC. Respiratory syncytial virus nonstructural protein 2 specifically inhibits type I interferon signal transduction. Virology. 2006; 344:328–39.

30). Elliott J, Lynch OT, Suessmuth Y, Qian P, Boyd CR, Burrows JF, et al. Respiratory syncytial virus NS1 protein degrades STAT2 by using the Elongin-Cullin E3 ligase. J Virol. 2007; 81:3428–36.

31). Goswami R, Majumdar T, Dhar J, Chattopadhyay S, Bandyopadhyay SK, Verbovetskaya V, et al. Viral degradasome hijacks mitochondria to suppress innate immunity. Cell Res. 2013; 23:1025–42.

32). Munir S, Le Nouen C, Luongo C, Buchholz UJ, Collins PL, Bukreyev A. Nonstructural proteins 1 and 2 of respiratory syncytial virus suppress maturation of human dendritic cells. J Virol. 2008; 82:8780–96.

33). Munir S, Hillyer P, Le Nouën C, Buchholz UJ, Rabin RL, Collins PL, et al. Respiratory syncytial virus interferon antagonist NS1 protein suppresses and skews the human T lymphocyte response. PLoS Pathog. 2011; 7:e1001336.

34). Qin L, Peng D, Hu C, Xiang Y, Zhou Y, Tan Y, et al. Differentiation of th subsets inhibited by nonstructural proteins of respiratory syncytial virus is mediated by ubiquitination. PLoS One. 2014; 9:e101469.

35). Atreya PL, Peeples ME, Collins PL. The NS1 protein of human respiratory syncytial virus is a potent inhibitor of minigenome transcription and RNA replication. J Virol. 1998; 72:1452–61.

36). Jin H, Zhou H, Cheng X, Tang R, Munoz M, Nguyen N. Recombinant respiratory syncytial viruses with deletions in the NS1, NS2, SH, and M2-2 genes are attenuated in vitro and in vivo. Virology. 2000; 273:210–8.
37). Tran KC, He B, Teng MN. Replacement of the respiratory syncytial virus nonstructural proteins NS1 and NS2 by the V protein of parainfluenza virus 5. Virology. 2007; 368:73–82.

38). Bitko V, Shulyayeva O, Mazumder B, Musiyenko A, Ramaswamy M, Look DC, et al. Nonstructural proteins of respiratory syncytial virus suppress premature apoptosis by an NF-kappaB-dependent, interferon-independent mechanism and facilitate virus growth. J Virol. 2007; 81:1786–95.
39). Liesman RM, Buchholz UJ, Luongo CL, Yang L, Proia AD, DeVincenzo JP, et al. RSV-encoded NS2 promotes epithelial cell shedding and distal airway obstruction. J Clin Invest. 2014; 124:2219–33.

Go to : 
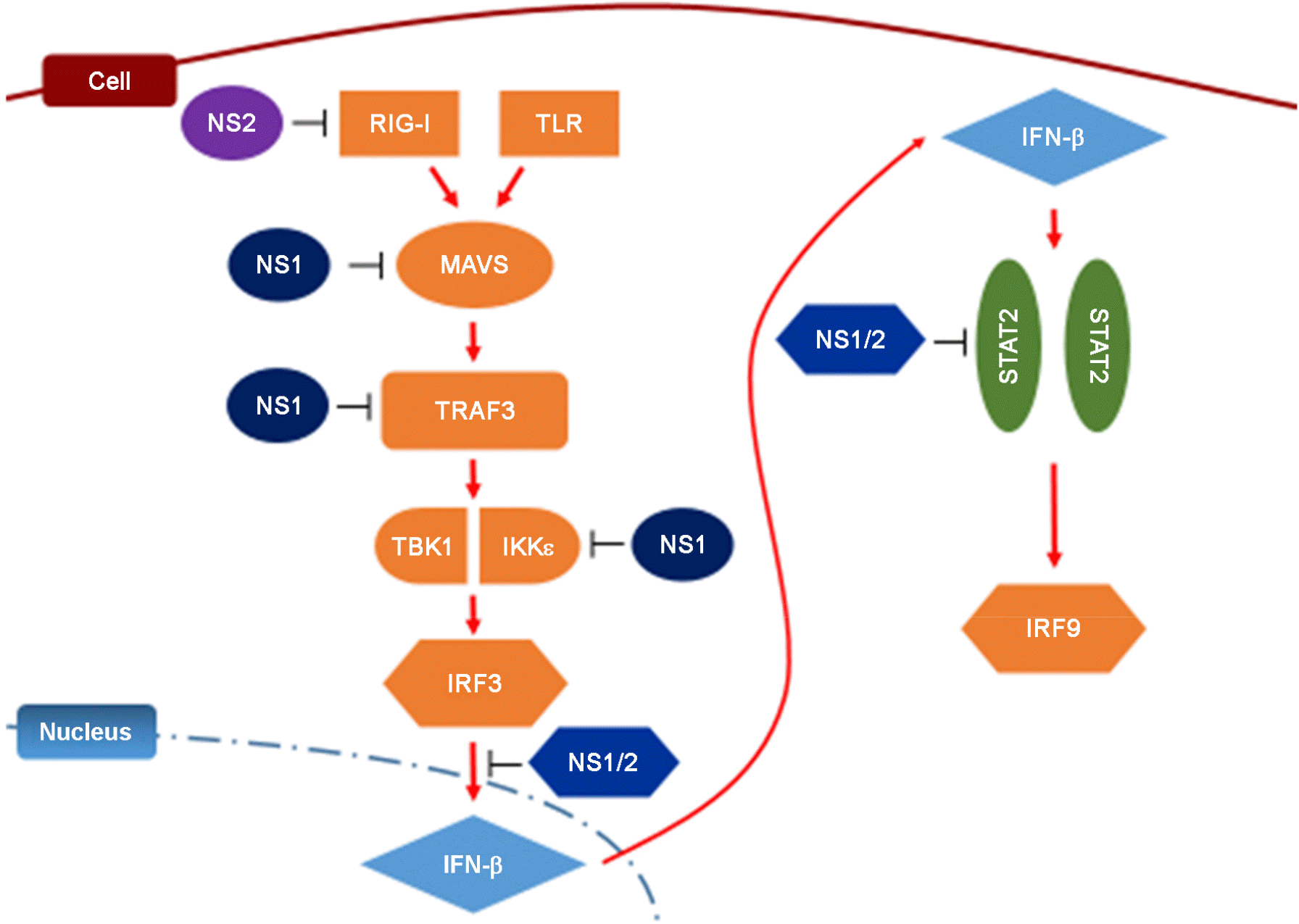 | Figure 1.Inhibition of antiviral interferon signaling pathway by RSV NS1 and NS2. NS1 and NS2 suppress type-I interferon signaling by targeting various key components of RIG-I (retinoic acid-inducible gene 1) and TLR (toll-like receptor) signaling pathways such as MAVS (mitochondrial antiviral signaling protein), TRAF3, IRF3 (interferon regulatory factor) and STAT2 (signal transducer and activator of transcription 2). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download