Abstract
It has been previously demonstrated that dystrophin is cleaved in the cardiac myocyte by the viral protease 2A following infection with Coxsackievirus B3 (CVB3). The viral protease 2A mediated cardiomyopathy can be prevented by inhibiting cleavage of dystrophin. However, it is less clear whether uncleaved dysdrophin have other heart protective effect in coxsackievirus infection. To address this, we generated a Balb/C background mouse that had a point mutation in dystrophin that prevents cleavage by protease 2A (KI). We show here that when mice expressing cleavage-resistant dystrophin were infected with CVB3, there was increased cardiac myocyte apoptosis. Bax and Bcl-XL mRNA ratio was significantly increased in KI mice heart compare to wild type mice heart. We found cleavage-resistant dystrophin induced the apoptosis related enzyme capspase-3 and caspase-8 activity. In addition, TUNEL stain was observed many TUNEL positive cardiac myocyte in KI mice heart compare to wild type mice heart (3.7% vs 0.3%). However, zVAD treatment for apoptosis blocking was significantly decreased myocardium damage and fibrosis in KI mice heart. These findings indicated that uncleaved dystrophin may have a critical role in cardiac myocyte viral propagation. Uncleaved dystrophin mutant induced cardiac myocyte apoptosis. It delayed coxsackievirus propagation in cardiac myocyte and could prevent cardiac myocyte death.
REFERENCES
1). Knowlton KU, Jeon ES, Berkley N, Wessely R, Huber S. A mutation in the puff region of VP2 attenuates the myocarditic phenotype of an infectious cDNA of the Woodruff variant of coxsackievirus B3. J Virol. 1996; 70:7811–8.

2). Badorff C, Lee GH, Lamphear BJ, Martone ME, Campbell KP, Rhoads RE, et al. Enteroviral protease 2A cleaves dystrophin: evidence of cytoskeletal disruption in an acquired cardiomyopathy. Nat Med. 1999; 5:320–6.

3). Gradi A, Imataka H, Svitkin YV, Rom E, Raught B, Morino S, et al. A novel functional human eukaryotic translation initiation factor 4G. Mol Cell Biol. 1998; 18:334–42.

4). Lamphear BJ, Yan R, Yang F, Waters D, Liebig HD, Klump H, et al. Mapping the cleavage site in protein synthesis initiation factor eIF-4 gamma of the 2A proteases from human Coxsackievirus and rhinovirus. J Biol Chem. 1993; 268:19200–3.

5). Castelló A, Alvarez E, Carrasco L. Differential cleavage of eIF4GI and eIF4GII in mammalian cells. Effects on translation. J Biol Chem. 2006; 281:33206–16.
6). Xiong D, Lee GH, Badorff C, Dorner A, Lee S, Wolf P, et al. Dystrophin deficiency markedly increases enterovirus-induced cardiomyopathy: a genetic predisposition to viral heart disease. Nat Med. 2002; 8:872–7.

7). Mavrogeni S, Papavasiliou A, Spargias K, Constandoulakis P, Papadopoulos G, Karanasios E, et al. Myocardial inflammation in Duchenne Muscular Dystrophy as a precipitating factor for heart failure: a prospective study. BMC Neurol. 2010; 10:33.

8). Lim BK, Peter AK, Xiong D, Narezkina A, Yung A, Dalton ND, et al. Inhibition of Coxsackievirus-associated dystrophin cleavage prevents cardiomyopathy. J Clin Invest. 2013; 123:5146–51.

9). Baboonian C, Davies MJ, Booth JC, McKenna WJ. Coxsackie B viruses and human heart disease. Curr Top Microbiol Immunol. 1997; 223:31–52.

10). Colston JT, Chandrasekar B, Freeman GL. Expression of apoptosis-related proteins in experimental coxsackievirus myocarditis. Cardiovasc Res. 1998; 38:158–68.

11). Peng T, Sadusky T, Li Y, Coulton GR, Zhang H, Archard LC. Altered expression of Bag-1 in Coxsackievirus B3 infected mouse heart. Cardiovasc Res. 2001; 50:46–55.

12). Kytö V, Lapatto R, Lakkisto P, Saraste A, Voipio-Pulkki LM, Vuorinen T, et al. Glutathione depletion and cardiomyocyte apoptosis in viral myocarditis. Eur J Clin Invest. 2004; 34:167–75.

13). Yun SH, Lee WG, Kim YC, Ju ES, Lim BK, Choi JO, et al. Antiviral activity of coxsackievirus B3 3C protease inhibitor in experimental murine myocarditis. J Infect Dis. 2012; 205:491–7.

14). Van Noorden CJ. The history of Z-VAD-FMK, a tool for understanding the significance of caspase inhibition. Acta Histochem. 2001; 103:241–51.

15). Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999; 13:1899–911.

16). Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995; 376:37–43.

17). Kawai C. From myocarditis to cardiomyopathy: mechanisms of inflammation and cell death: learning from the past for the future. Circulation. 1999; 99:1091–100.
18). Suzuki K, Kostin S, Person V, Elsässer A, Schaper J. Time course of the apoptotic cascade and effects of caspase inhibitors in adult rat ventricular cardiomyocytes. J Mol Cell Cardiol. 2001; 33:983–94.

19). Huber SA. T cells expressing the gamma delta T cell receptor induce apoptosis in cardiac myocytes. Cardiovasc Res. 2000; 45:579–87.
Figure 1.
Real-time PCR for apoptosis detection. Bax and Bcl-XL expression was confirmed by real-time PCR. After 4 and 8 days CVB3 infection, WT (n=5) and KI (n=5) mice heart was collected and RNA was extracted for rt-PCR. Bax level were significantly increased in KI mice heart compare to WT mice heart. The ratio of Bax/Bcl-XL was significantly increased at day4 and 8 CVB3 post-infection in KI mice heart. Data are presented as the mean plus or minus the standard error of the mean from 3 independent experiments (Mean ± S.E.M, **p < 0.01, *p < 0.05, each data point is represented on graphs).

Figure 2.
Caspase-3 and 8 protease activity. Caspase-3 and 8 protease activity was measured by PPAR and caspase-3 cleavage site mimic substrate Ac-DEVD-pND (Acetyl-Asp-Glu-Val-Asp-p-nitroanilide) and Ac-IETD-pND (Acetyl-Ile-Glu-Thr-Asp-p-nitroanilide). Caspase-3 and 8 protease activity was significantly higher in KI mice (n=8) heart compare to WT mice (n=6) heart (caspase-3, 2.5±0.2-fold vs 3.3±0.1-fold: caspase-8, 1.2±0.1-fold vs 1.7±0.1-fold; fold induction compare to uninfected control). Mean ± S.E.M, ***p < 0.01, each data point is represented on graphs.
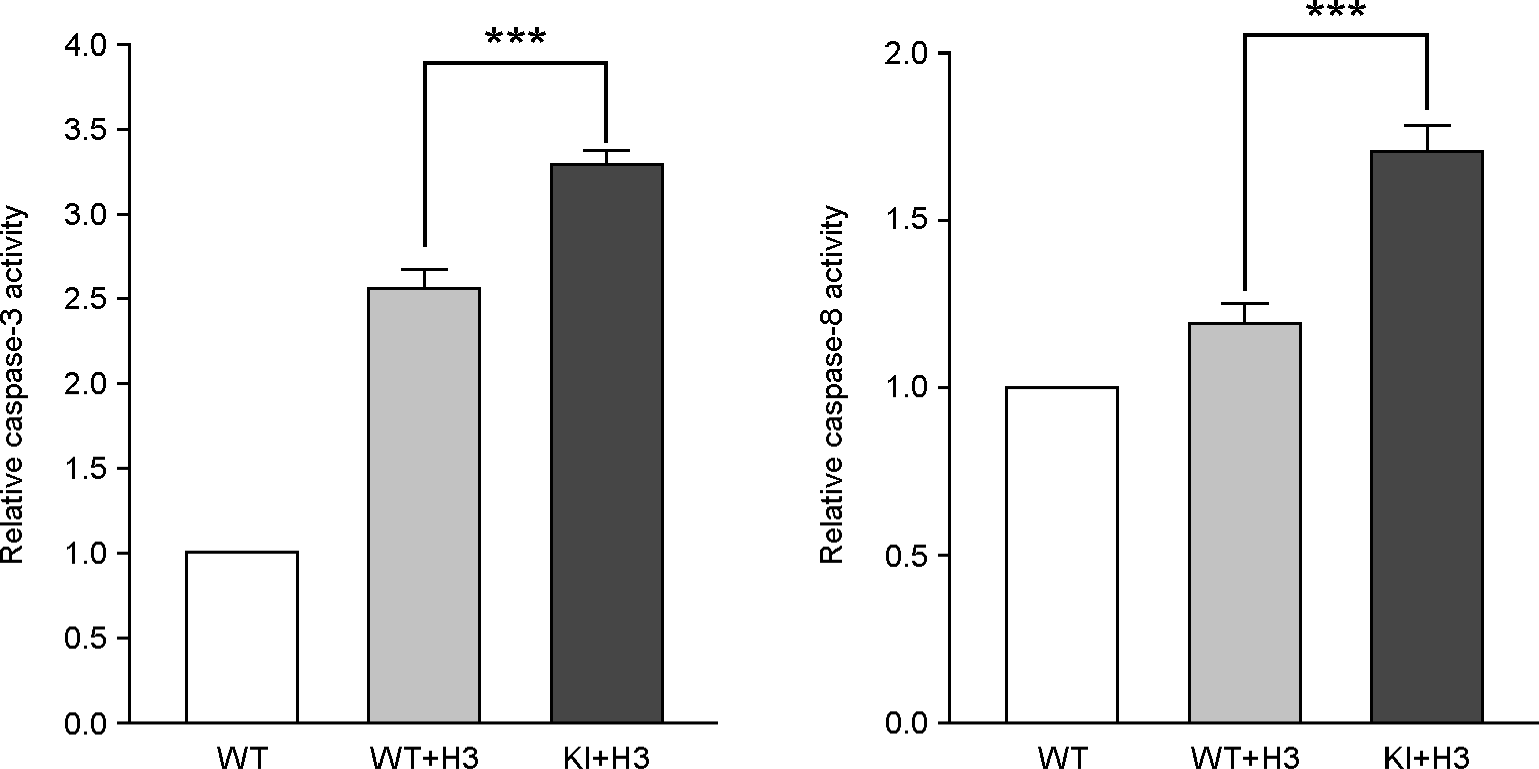
Figure 3.
Increase TUNEL stain positive cardiac myocyte. TUNEL stain was performed in CVB3 infected WT and KI mice heart to detect apoptosis positive cardiac myocyte. TUNEL positive cells (arrow) were significantly higher detected in KI mice heart compare to WT mice heart (3.5±0.4 % vs 0.3±0.1%). Inhibition of dystrophin cleavage induced apoptosis in CVB3 murine myocarditis model. Mean ± S.E.M, ***p < 0.01, each data point is represented on graphs.
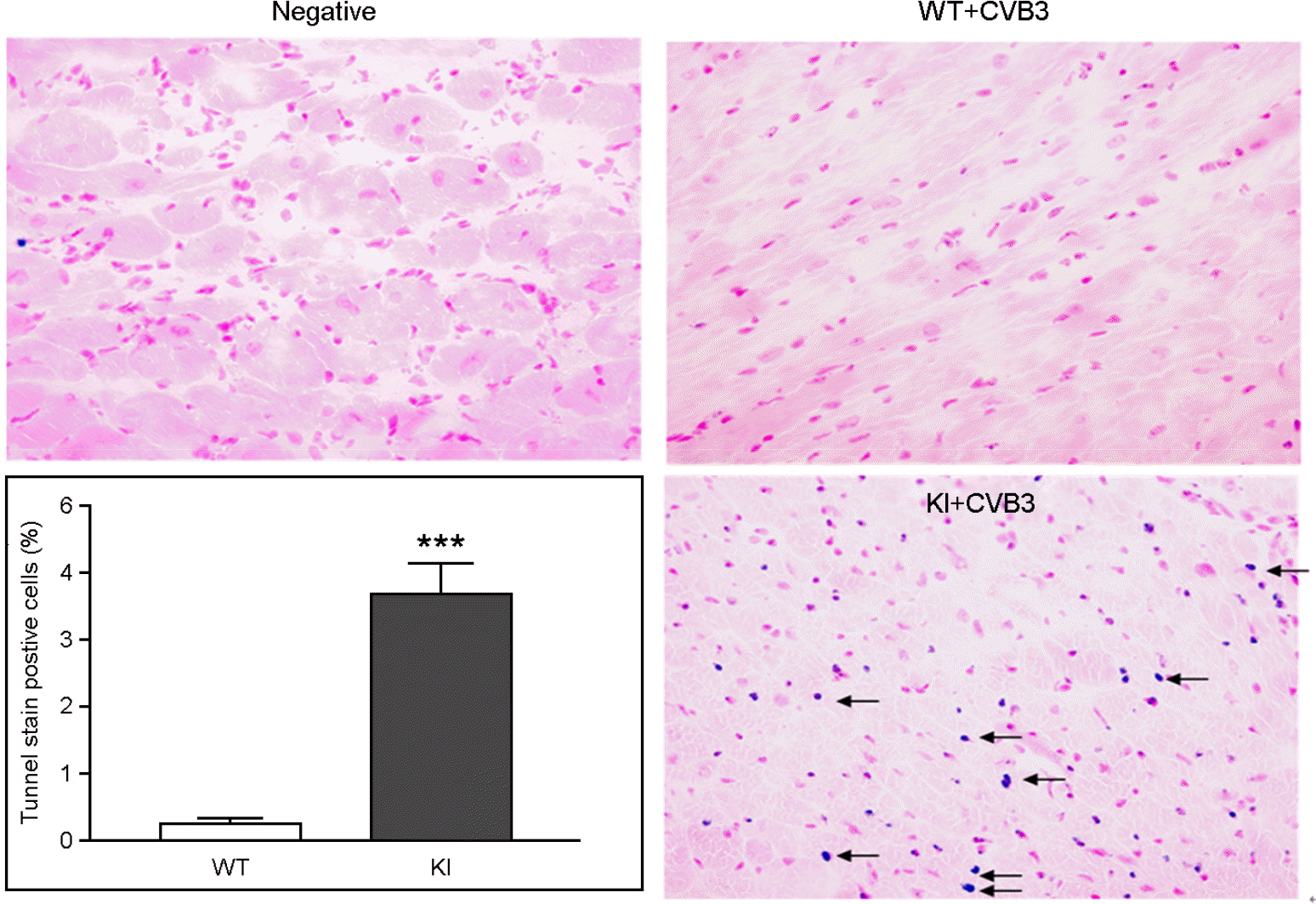
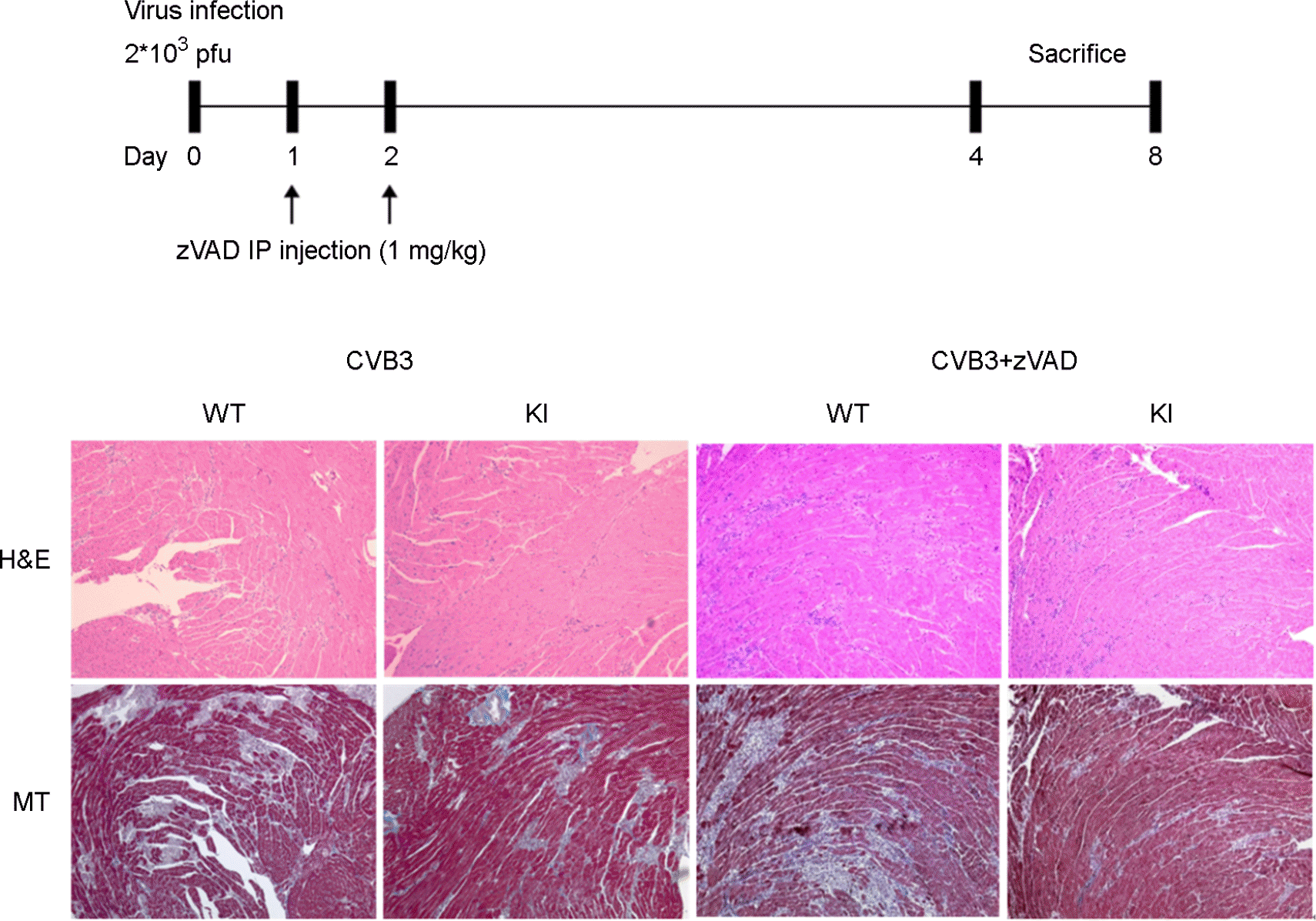
Figure 4.
Decrease cardiac myocyte inflammation and fibrosis. Schematic diagram of animal experiment for zVAD treat in murine myocarditis model. Uncleaved dystrophin was effectively blocked enterovirus replication and propagation in the heart. Inflammation (H&E) and fibrosis (MT) were decreased in KI mice heart compare to WT mice heart. In addition, apoptosis inhibitor (zVAD) treatment with CVB3 was dramatically decreased heart inflammation and fibrosis in KI mice heart.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download