Abstract
Two Gram-positive rod strains isolated from the healthy vagina of a woman were tested for the possibility as probiotics. One strain was identified as Steroidobacter denitrificans (YH1) and the other as Lactobacillus crispatus (YH2) by 16S rRNA partial sequencing. The Casman agar and Man-Rogosa-Sharpe (MRS) agar were mixed in same quantity, supplemented with 5% human rbc lysate (CMB agar). The Wilkins-Chalgren agar and MRS agar were mixed in same quantity (WCM agar). Gardnerella vaginalis was cultured in Casman broth, supplemented with 5% human rbc lysate and 1,000 x-diluted with normal saline. Bacteroides fragilis, Mobiluncus mulieris and Peptostreptococcus asaccharolyticus were cultured in Wilkins-Chalgren anaerobe broth and 2,000x-diluted. S. denitrificans YH1 and L. crispatus YH2 were cultured in MRS broth anaerobically and 100x-diluted. The diluted suspensions of B. fragilis, M. mulieris and P. asaccharolyticus were inoculated on WCM agar and G. vaginalis on CMB agar by cotton swabs. Ten μl aliquots of YH1 and YH2 were inoculated on the center of WCM agar and CMB agar. The growth inhibition zone diameters of B. fragilis, G. vaginalis, M. mulieris and P. asaccharolyticus by YH1 were 35 mm, 35 mm, 25 mm and 60 mm. The inhibition diameters by YH2 were 25 mm, 30 mm, 20 mm and 40 mm, respectively. These results implicate that S. denitrificans YH1 can be the stronger probiotics for the treatment of bacterial vaginosis than L. crispatus, compared inhibition zone diameters by YH1 and YH2.
REFERENCES
1). Singh S, Goswami P, Singh R, Heller KJ. Application of molecular identification tools for Lactobacillus, with a focus on discrimination between closely related species: A review. Lwt-Food Sci Tech. 2009; 42:448–57.

2). Joesoef MR, Schmid G. Bacterial vaginosis. Clin Evid. 2005; 13:1968–78.
3). Antonio M, Petrina M, Meyn L, Hillier S. Lactobacillus crispatus colonisation reduces risk of bacterial vaginosis (BV) acquisition. Sex Transmit Infect. 2011; 87:A304–5.
4). Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983; 74:14–22.
5). Yang NW, Sin SH, Chang JS. Diagnosis of bacterial vaginosis with relation to isolation of Gardnerella vaginalis. J Bactriol Virol. 2002; 32:109–14.
6). Yang NW, Kim JM, Choi GJ, Jang SJ. Development and evaluation of the quick anaero-system-A new disposable anaerobic culture system. Korean J Lab Med. 2010; 30:133–7.

7). Yang NW, Lim Y, Sin SH. Drug-resistant profiles of clinical isolates of Gardnerella vaginalis on Columbia agar base supplemented with human erythrocyte lysate and horse serum. Infect Chemother. 2003; 35:86–90.
8). Dawson SG, Harris JR. Gardnerella vaginalis and nonspecific vaginitis. Br J Hosp Med. 1983; 29:28–37.
9). Borchardt KA, Adly BS, Smith RF, Eapen J, Beal CB. Importance of Gardnerella vaginalis as an aetiological agent in bacterial vaginosis. Genitourin Med. 1989; 65:285.
10). Machado A, Jefferson KK, Cerca N. Interactions between Lactobacillus crispatus and bacterial vaginosis (BV)-associated bacterial species in initial attachment and biofilm formation. Int J Mol Sci. 2013; 14:12004–12.
11). Chang CE, Kim SC, So JS, Yun HS. Cultivation of Lactobacillus crispatus KLB46 isolated from human vagina. Biotechnol Bioprocess Eng. 2001; 6:128–32.
12). Fahrbach M, Kuever J, Remesch M, Huber BE, Kämpfer P, Dott W, et al. Steroidobacter denitrificans gen. nov., sp. nov., a steroidal hormone-degrading γ-proteobacterium. Int J Syst Evol Microbiol. 2008; 58:2215–23.
13). Chiang YR, Fang JY, Ismail W, Wang PH. Initial steps in anoxic testosterone degradation by Steroidobacter denitrificans. Microbiology. 2010; 156:2253–9.
14). Wang PH, Leu YL, Ismail W, Tang SL, Tsai CY, Chen HJ, et al. Anaerobic and aerobic cleavage of the steroid core ring structure by Steroidobacter denitrificans. J Lipid Res. 2013; 54:1493–504.
Figure 1.
Sample A shows the 99% similarity to Steroidobacter denitrificans accession No. EF605262 in the unrooted neighbour-joining phylogenetic tree based on 16s rRNA gene sequence comparison.
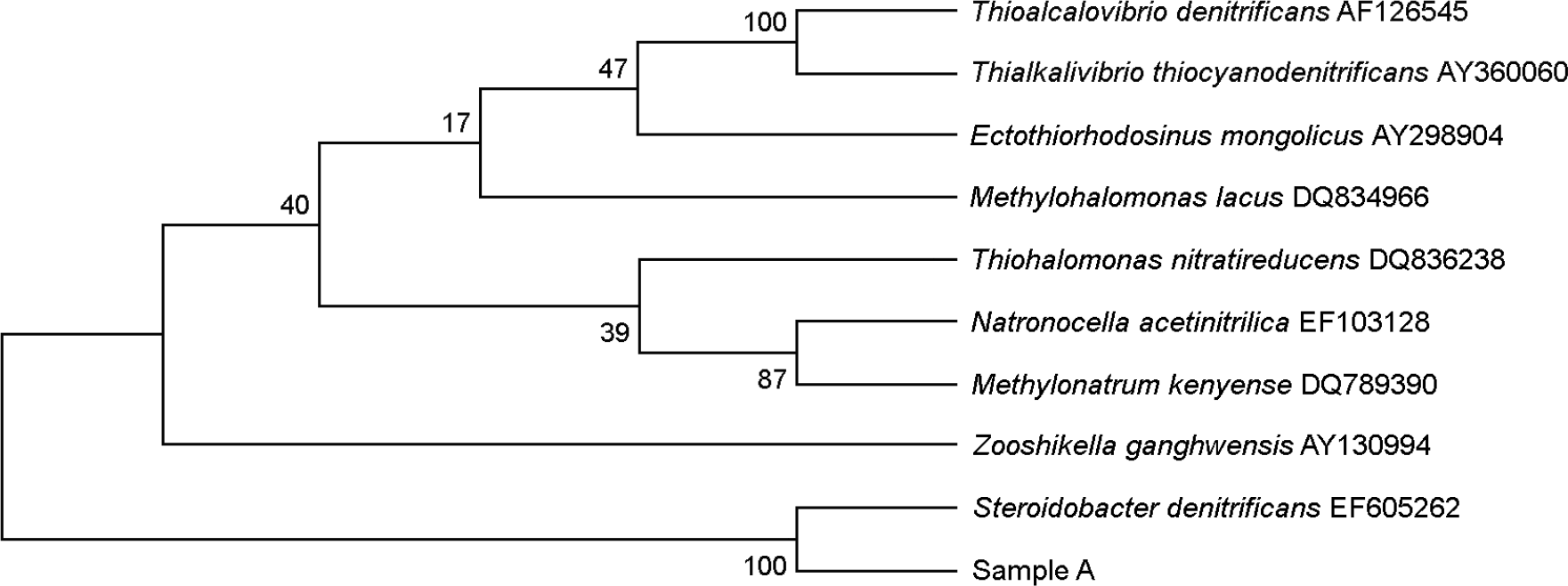
Figure 2.
Sample B shows the 99% similarity to Lactobacillus crispatus accession No. AF257097 in the unrooted neighbour-joining phylogenetic tree based on 16s rRNA gene sequence comparison.
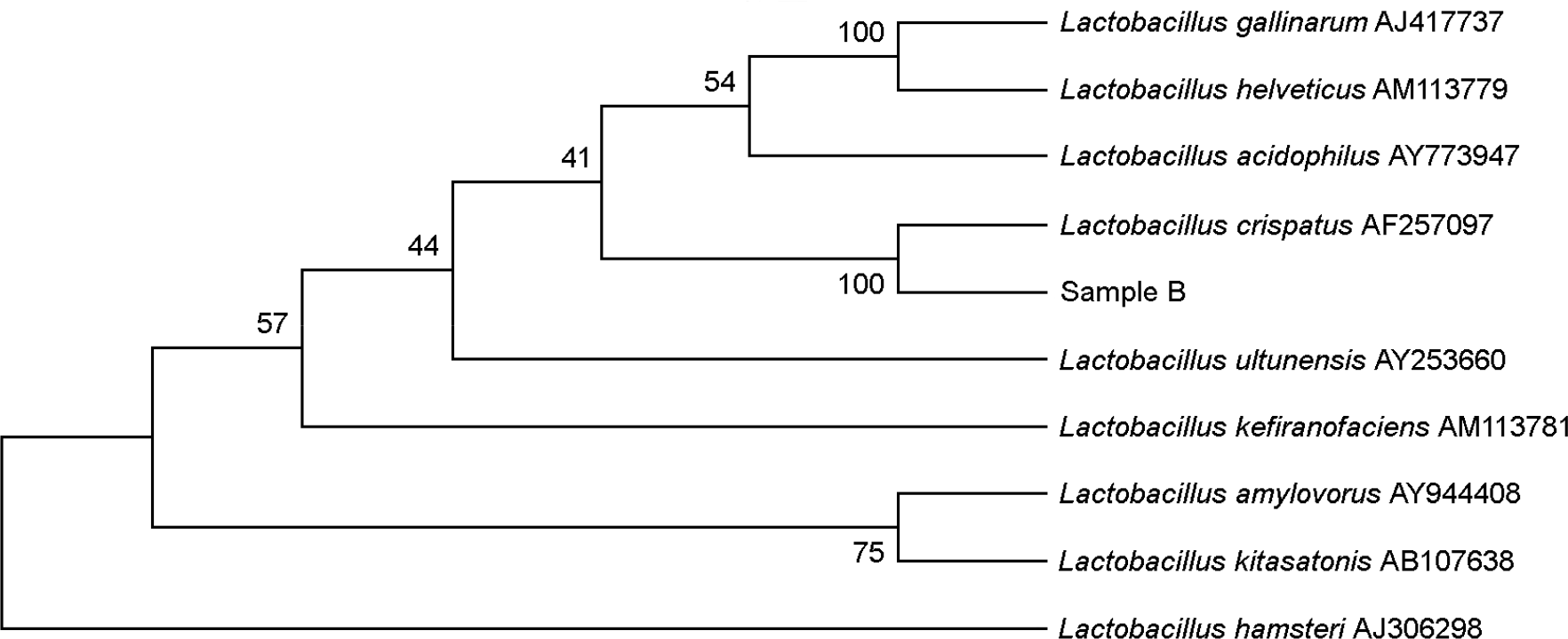
Figure 3.
Growth inhibition zones by S. denitrificans YH1. A, B, C, and D is B. fragilis, G. vaginalis, M. mulieris, and P. asaccharolyticus in regular order.
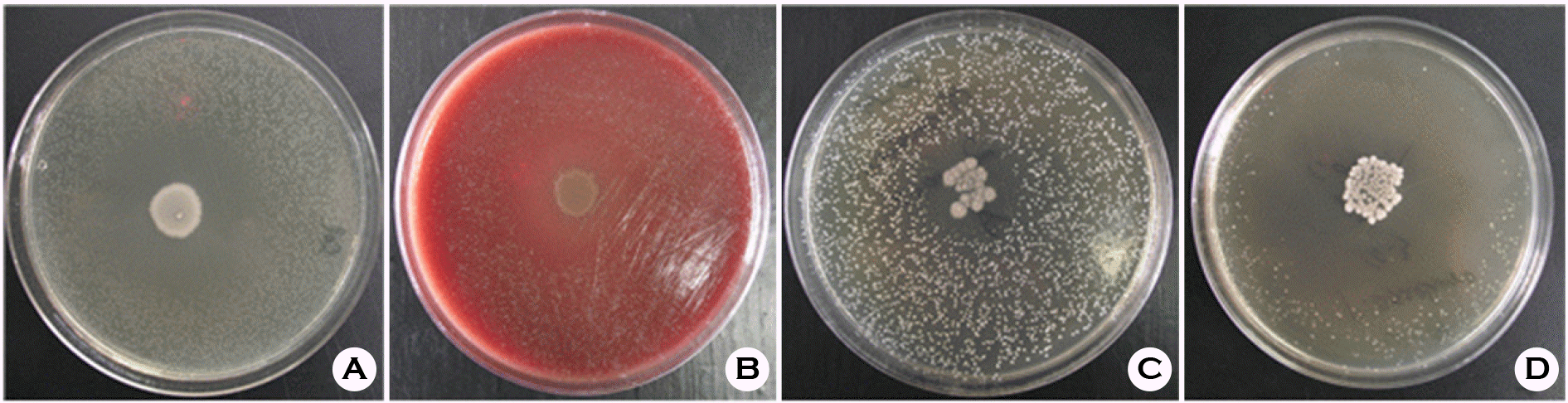
Figure 4.
Growth inhibition zones by L. crispatus YH2. A, B, C, and D is B. fragilis, G. vaginalis, M. mulieris, and P. asaccharolyticus in regular order.
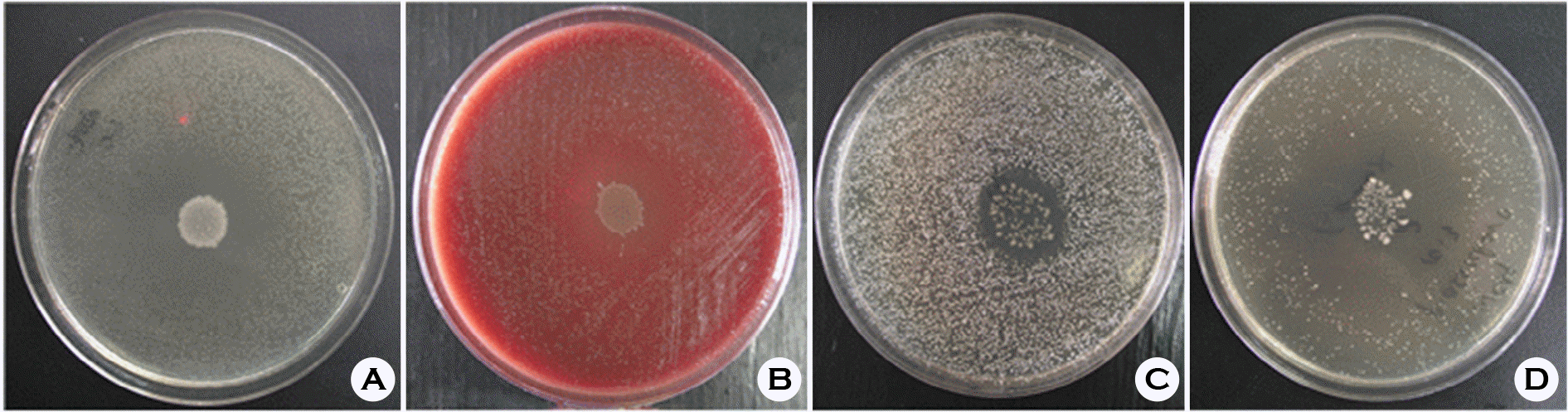
Table 1.
Carbohydrate biochemical test results of L. crispatus YH2 by API CHL kit
Table 2.
Carbohydrate biochemical test results of S. denitrificans YH1 by API CHL kit
Table 3.
Enzyme activity results of Steroidobacter denitrificans YH1 by API ZYM kit




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download