Abstract
Human immunodeficiency virus type 1 (HIV-1)-infected cells respond to the infection with different outcomes depending on their cell type. The interplay of cellular and viral proteins is a key player of differences in virus replication and disease progression. Myeloid cells, including monocytes, macrophages, and myeloid dendritic cells (mDCs) play a crucial role in the transmission and pathogenesis of HIV. The viral protein Tat, which is the viral transcriptional activator, modulates the expression of both HIV and cellular genes in these myeloid cells. This review will focus on recent advances on the interplay between HIV and myeloid cells and will discuss how this interaction may contribute to HIV pathogenesis. A better understanding of the pathogenesis of HIV disease will provide us with the scientific rationale for novel approaches to prevention.
Go to : 
REFERENCES
1). Cohen MS, Shaw GM, McMichael AJ, Haynes BF. Acute HIV-1 infection. N Engl J Med. 2011; 364:1943–54.

2). Wu Y, Marsh JW. Selective transcription and modulation of resting T cell activity by preintegrated HIV DNA. Science. 2001; 293:1503–6.

3). Gartner S, Markovits P, Markovitz DM, Kaplan MH, Gallo RC, Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986; 233:215–9.

4). Brown CR, Czapiga M, Kabat J, Dang Q, Ourmanov I, Nishimura Y, et al. Unique pathology in simian immunodeficiency virus-infected rapid progressor macaques is consistent with a pathogenesis distinct from that of classical AIDS. J Virol. 2007; 81:5594–606.

5). Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006; 124:783–801.

6). Mir KD, Mavigner M, Silvestri G. The myeloid cytokine network in AIDS pathogenesis. Cytokine Growth Factor Rev. 2012; 23:223–31.

7). Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010; 327:656–61.

8). Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010; 11:373–84.

9). Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004; 5:987–95.

10). Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006; 12:1365–71.

11). Gringhuis SI, van der Vlist M, van den Berg LM, den Dunnen J, Litjens M, Geijtenbeek TB. HIV-1 exploits innate signaling by TLR8 and DC-SIGN for productive infection of dendritic cells. Nat Immunol. 2010; 11:419–26.

12). Hernández JC, Arteaga J, Paul S, Kumar A, Latz E, Urcuqui-Inchima S. Up-regulation of TLR2 and TLR4 in dendritic cells in response to HIV type 1 and coinfection with opportunistic pathogens. AIDS Res Hum Retroviruses. 2011; 27:1099–109.

13). Wu L, KewalRamani VN. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat Rev Immunol. 2006; 6:859–68.

14). St Gelais C, Coleman CM, Wang JH, Wu L. HIV-1 Nef enhances dendritic cell-mediated viral transmission to CD4+ T cells and promotes T-cell activation. PLoS One. 2012; 7:e34521.

15). Jolly C, Kashefi K, Hollinshead M, Sattentau QJ. HIV-1 cell to cell transfer across an Env-induced, actindependent synapse. J Exp Med. 2004; 199:283–93.

16). Goujon C, Malim MH. Characterization of the alpha interferon-induced postentry block to HIV-1 infection in primary human macrophages and T cells. J Virol. 2010; 84:9254–66.

17). Pertel T, Reinhard C, Luban J. Vpx rescues HIV-1 transduction of dendritic cells from the antiviral state established by type 1 interferon. Retrovirology. 2011; 8:49.

18). Coleman CM, Wu L. HIV interactions with monocytes and dendritic cells: viral latency and reservoirs. Retrovirology. 2009; 6:51.

19). Belz GT, Nutt SL. Transcriptional programming of the dendritic cell network. Nat Rev Immunol. 2012; 12:101–13.

20). Reinhart TA, Fallert BA, Pfeifer ME, Sanghavi S, Capuano S 3rd, Rajakumar P, et al. Increased expression of the inflammatory chemokine CXC chemokine ligand 9/monokine induced by interferon-γ in lymphoid tissues of rhesus macaques during simian immunodeficiency virus infection and acquired immunodeficiency syndrome. Blood. 2002; 9:3119–28.

21). Rich EA, Chen IS, Zack JA, Leonard ML, O'Brien WA. Increased susceptibility of differentiated mononuclear phagocytes to productive infection with human immunodeficiency virus-1 (HIV-1) J Clin Invest. 1992; 89:176–83.
22). Mikovits JA, Raziuddin , Gonda M, Ruta M, Lohrey NC, Kung HF, et al. Negative regulation of human immune deficiency virus replication in monocytes. Distinctions between restricted and latent expression in THP-1 cells. J Exp Med. 1990; 171:1705–20.
23). Sabatier JM, Vives E, Mabrouk K, Benjouad A, Rochat H, Duval A, et al. Evidence for neurotoxic activity of tat from human immunodeficiency virus type 1. J Virol. 1991; 65:961–7.

24). Nakajima K, Martínez-Maza O, Hirano T, Breen EC, Nishanian PG, Salazar-Gonzalez JF, et al. Induction of IL-6 (B cell stimulatory factor-2/IFN-beta 2) production by HIV. J Immunol. 1989; 142:531–6.
25). Embretson J, Zupancic M, Ribas JL, Burke A, Racz P, Tenner-Racz K, et al. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993; 362:359–62.

26). Williams K, Ulvestad E, Antel J. Immune regulatory and effector properties of human adult microglia studies in vitro and in situ. Adv Neuroimmunol. 1994; 4:273–81.
27). Hufert FT, Schmitz J, Schreiber M, Schmitz H, Rácz P, von Laer DD. Human Kupffer cells infected with HIV-1 in vivo. J Acquir Immune Defic Syndr. 1993; 6:772–7.
28). Pearce TE, Nowakowski M, Eden E, Huang ZB, Steiner P, Shahabuddin M, et al. Uniform detection of HIV-1 in alveolar macrophages of pediatric but not adult AIDS patients. J Leukoc Biol. 1993; 53:722–6.

29). Orenstein JM, Fox C, Wahl SM. Macrophages as a source of HIV during opportunistic infections. Science. 1997; 276:1857–61.

30). Sharova N, Swingler C, Sharkey M, Stevenson M. Macrophages archive HIV-1 virions for dissemination in trans. EMBO J. 2005; 24:2481–9.

31). Ho DD, Rota TR, Hirsch MS. Infection of monocyte/macrophages by human T lymphotropic virus type III. J Clin Invest. 1986; 77:1712–15.

32). Ackerman AL, Cresswell P. Cellular mechanisms governing cross-presentation of exogenous antigens. Nat Immunol. 2004; 5:678–84.

33). Brown A, Gartner S, Kawano T, Benoit N, Cheng-Mayer C. HLA-A2 down-regulation on primary human macrophages infected with an M-tropic EGFP-tagged HIV-1 reporter virus. J Leukoc Biol. 2005; 78:675–85.

34). Shen R, Richter HE, Clements RH, Novak L, Huff K, Bimczok D, et al. Macrophages in vaginal but not intestinal mucosa are monocyte-like and permissive to human immunodeficiency virus type 1 infection. J Virol. 2009; 83:3258–67.

35). Santa-Marta M, de Brito PM, Godinho-Santos A, Goncalves J. Host factors and HIV-1 replication: Clinical Evidence and Potential Therapeutic Approaches. Front Immunol. 2013; 4:343.

36). Groot F, Welsch S, Sattentau QJ. Efficient HIV-1 transmission from macrophages to T cells across transient virological synapses. Blood. 2008; 111:4660–3.

37). Laguette N, Sobhian B, Casartelli N, Ringeard M, Chable-Bessia C, Ségéral E, et al. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature. 2011; 474:654–7.

38). Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008; 451:425–30.

39). Kaushik R, Zhu X, Stranska R, Wu Y, Stevenson M. A cellular restriction dictates the permissivity of non-dividing monocytes/macrophages to lentivirus and gammaretrovirus infection. Cell Host Microbe. 2009; 6:68–80.

40). Lahouassa H, Daddacha W, Hofmann H, Ayinde D, Logue EC, Dragin L, et al. SAMHD1 restricts the replication of human immunodeficiency virus type 1 by depleting the intracellular pool of deoxynucleoside triphosphates. Nat Immunol. 2012; 13:223–8.

41). Civas A, Island ML, Génin P, Morin P, Navarro S. Regulation of virus-induced interferon-A genes. Biochimie. 2002; 84:643–54.

42). Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005; 5:375–86.

43). Francis M, Fan XS, Meltzer MS. Loss ability to produce IFN-alpha in response to HIV-1 as monocytes differentiate into macrophages. Induction through a mechanism independent of double-stranded RNA. J Immunol. 1996; 156:2481–7.
44). Schlee M, Hartmann E, Coch C, Wimmenauer V, Janke M, Barchet W, et al. Approaching the RNA ligand for RIG-I? Immunol Rev. 2009; 227:66–74.

45). Yan N, Regalado-Magdos AD, Stiggelbout B, Lee-Kirsch MA, Lieberman J. The cytosolic exonuclease TREX1 inhibits the innate immune response to human immunodeficiency virus type 1. Nat Immunol. 2010; 11:1005–13.

46). Marsili G, Remoli AL, Sgarbanti M, Perrotti E, Fragale A, Battistini A. HIV-1, interferon and the interferon regulatory factor system: an interplay between induction, antiviral responses and viral evasion. Cytokine Growth Factor Rev. 2012; 23:255–70.

47). Jacquelin B, Mayau V, Targat B, Liovat AS, Kunkel D, Petitjean G, et al. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest. 2009; 119:3544–55.

48). Zhou Q, Yik JH. The Yin and Yang of P-TEFb Regulation: implications for human immunodeficiency virus gene expression and global control of cell growth and differentiation. Microbiol Mol Biol Rev. 2006; 70:646–59.

49). Rotger M, Dang KK, Fellay J, Heinzen EL, Feng S, Descombes P, et al. Genome-wide mRNA expression correlates of viral control in CD4 T-cells from HIV-1-infected individuals. PLoS Pathog. 2010; 6:e1000781.

50). Kameoka M, Morgan M, Binette M, Russell RS, Rong L, Guo X, et al. The Tat protein of human immunodeficiency virus type 1 (HIV-1) can promote placement of tRNA primer onto viral RNA and suppress later DNA polymerization in HIV-1 reverse transcription. J Virol. 2002; 76:3637–45.

51). Li L, Li HS, Pauza CD, Bukrinsky M, Zhao RY. Roles of HIV-1 auxiliary proteins in viral pathogenesis and host-pathogen interactions. Cell Res. 2005; 15:923–34.

52). Gandhi N, Saiyed Z, Thangavel S, Rodriguez J, Rao KV, Nair MP. Differential effects of HIV type 1 clade B and clade C Tat protein on expression of proinflammatory and antiinflammatory cytokines by primary monocytes. AIDS Res Hum Retroviruses. 2009; 25:691–9.

53). Kim N, Kukkonen S, Martinez-Viedma Mdel P, Gupta S, Aldovini A. Tat engagement of p38 MAP kinase and IRF7 pathways leads to activation of interferon-stimulated genes in antigen-presenting cells. Blood. 2013; 121:4090–100.

54). Kim N, Dabrowska A, Jenner RG, Aldovini A. Human and simian immunodeficiency virus-mediated up-regulation of the apoptotic factor TRAIL occurs in antigen-presenting cells from AIDS-susceptible but not from AIDS-resistant species. J Virol. 2007; 81:7584–97.

55). Ju SM, Song HY, Lee JA, Lee SJ, Choi SY, Park J. Extracellular HIV-1 Tat up-regulates expression of matrix metalloproteinase-9 via a MAPK-NF-kappaB dependent pathway in human astrocytes. Exp Mol Med. 2009; 41:86–93.
56). Dabrowska A, Kim N, Aldovini A. Tat-induced FOXO3a is a key mediator of apoptosis in HIV-1-infected human CD4+ T lymphocytes. J Immunol. 2008; 181:8460–77.
57). Kim N, Kukkonen S, Gupta S, Aldovini A. Association of Tat with promoters of PTEN and PP2A subunits is key to transcriptional activation of apoptotic pathways in HIV-infected CD4+ T cells. PLoS Pathog. 2010; 6:e1001103.

58). Van Grevenynghe J, Procopio Fa, He Z, Chomont N, Riou C, Zhang Y, et al. Transcription Factor FOXO3a Controls The Persistence Of Memory Cd4 (+) T Cells During HIV Infection. Nat Med. 2008; 14:266–74.
Go to : 
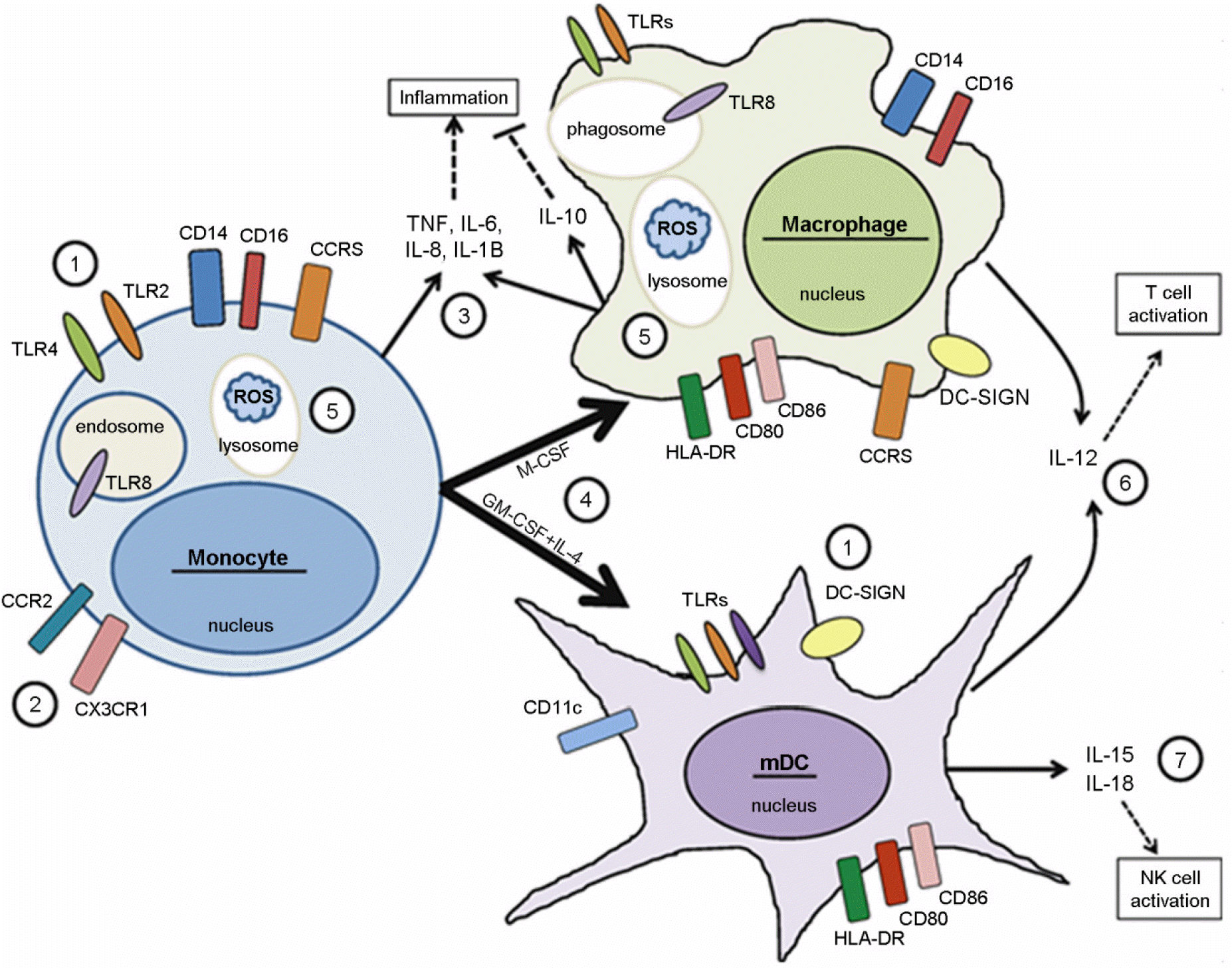 | Figure 1.Overview of myeloid cell functions. Monocytes, macrophages, and myeloid dendritic cells (mDCs) express multiple receptors to recognize microbes, including toll-like receptors (TLRs), and c-type lectin receptors such as DC-SIGN (1). Chemokine receptors such as CCR2 and CX3CR1 direct the migration of blood monocytes to sites of inflammation (2), where they contribute to the pro-inflammatory cytokine milieu through the production of TNF-α, IL-6, IL-8, and IL-1β (3). Once present in tissues, monocytes are influenced by local signals to differentiate into either macrophages or mDCs. In vitro, M-CSF directs monocyte differentiation into macrophages while GM-CSF + IL-4 results in differentiation into mDCs(4). Tissue resident macrophages are also capable of producing pro-inflammatory cytokines as well as ample amounts of IL-10, an anti-inflammatory cytokine important for resolving local inflammation (3). Both monocytes and macrophages are capable of phagocytosis and intracellular killing of microbes through the production of reactive oxygen species (ROS) and reactive nitrogen species (5). Activated macrophages and mDCs secrete IL-12, a cytokine involved in the activation and differentiation of T cells (6). Activated mDCs also stimulate NK cell activation via the production of IL-15 and IL-18 (7). Adapted from “The myeloid cytokine network in AIDS pathogenesis”, by Mr et al., 2012, Cytokine Growth Factor Rev, 23, 224 as a reference 6 in the text. |
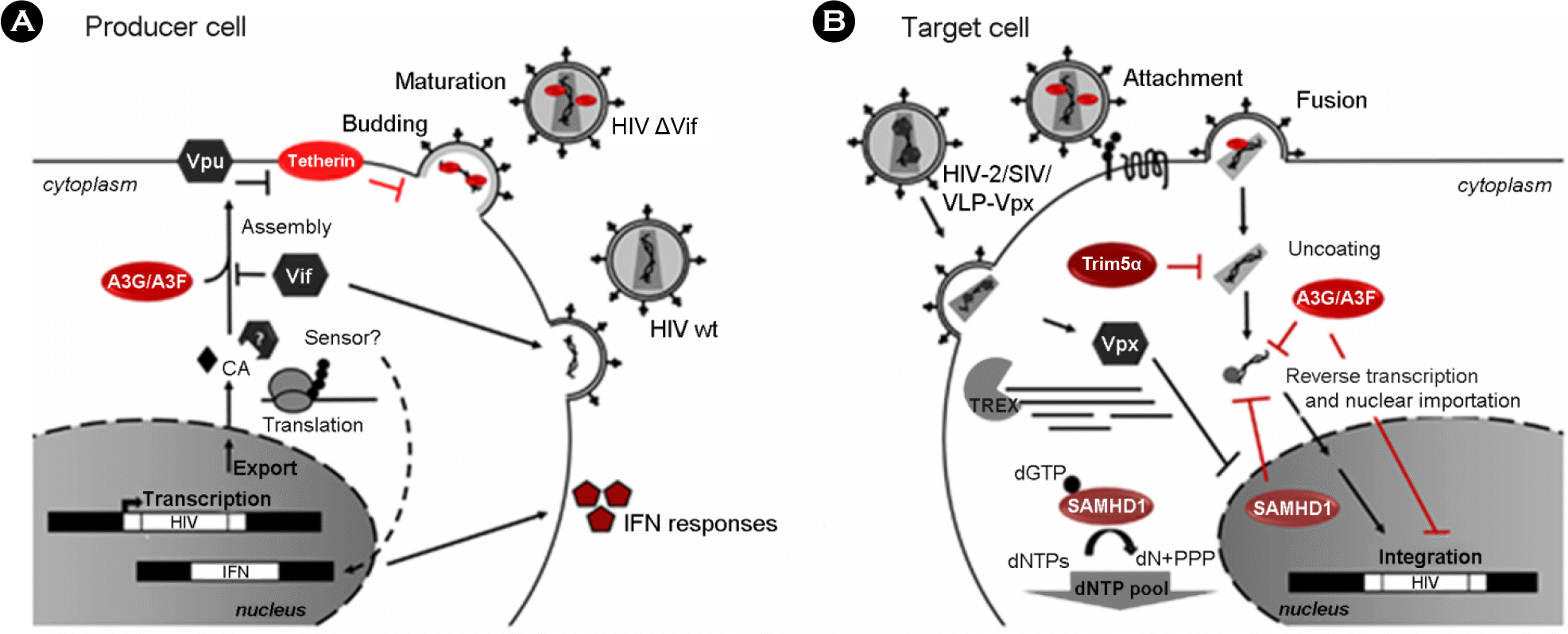 | Figure 2.Host restriction factors and their action during HIV-1 replication. Schematic representation of (A) HIV-1-infected producer cell, and (B) HIV-1 target cell. Cellular restriction factors are represented by red ovals, and viral counterpartners are represented by gray hexagons. Black arrows represent the course of viral replication and actions. Broken arrows represent inhibition. Question marks (?) represent unresolved questions. Adapted from “Host factors and HIV-1 replication”, by Goncalves et al., 2013, Front Immunol, 4, 343 as a reference 35 in the text. |
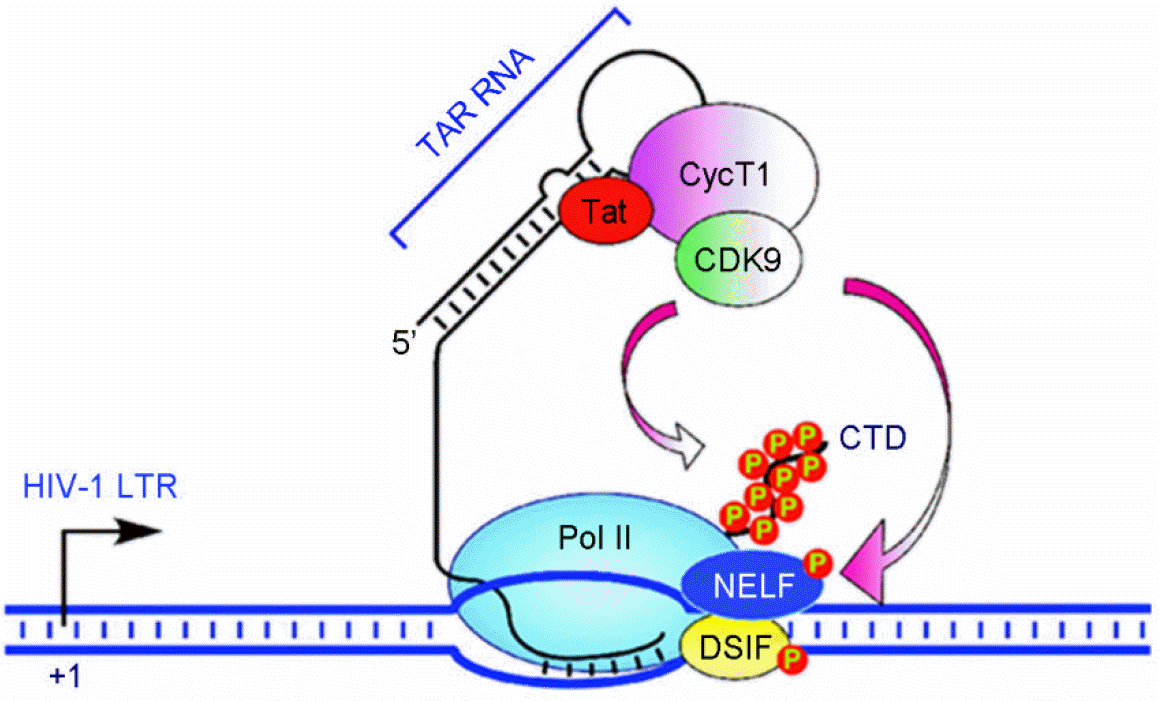 | Figure 3.Schematic of the pathway triggered by Tat and leading to activation of ISGs by Tat in APCs infected by HIV. (A) Tat triggers signaling pathways that activate ISGs by associating with MAPK kinases MAP2K3 (MKK3) and MAP2K6 (MKK6), which in turn activate p38 MAPK and STAT1, and with IRF7 in APCs infected by HIV. (B) Activation of MKK3, MKK6, and IRF7 leads to expression of many ISGs (shown here in connection with MKK3, MKK6, and IRF7), whose product can positively impact T-cell immunoactivation and negatively impact virus production. Bold labeling indicates genes found upregulated by HIV and Tat in APC. Adapted from “Tat engagement of p38 MAP kinase and IRF7 pathways leads to activation of interferon-stimulated genes in antigen-presenting cells”, by Kim et al., 2013, Blood, 121, 4090 as a reference 53 in the text. |
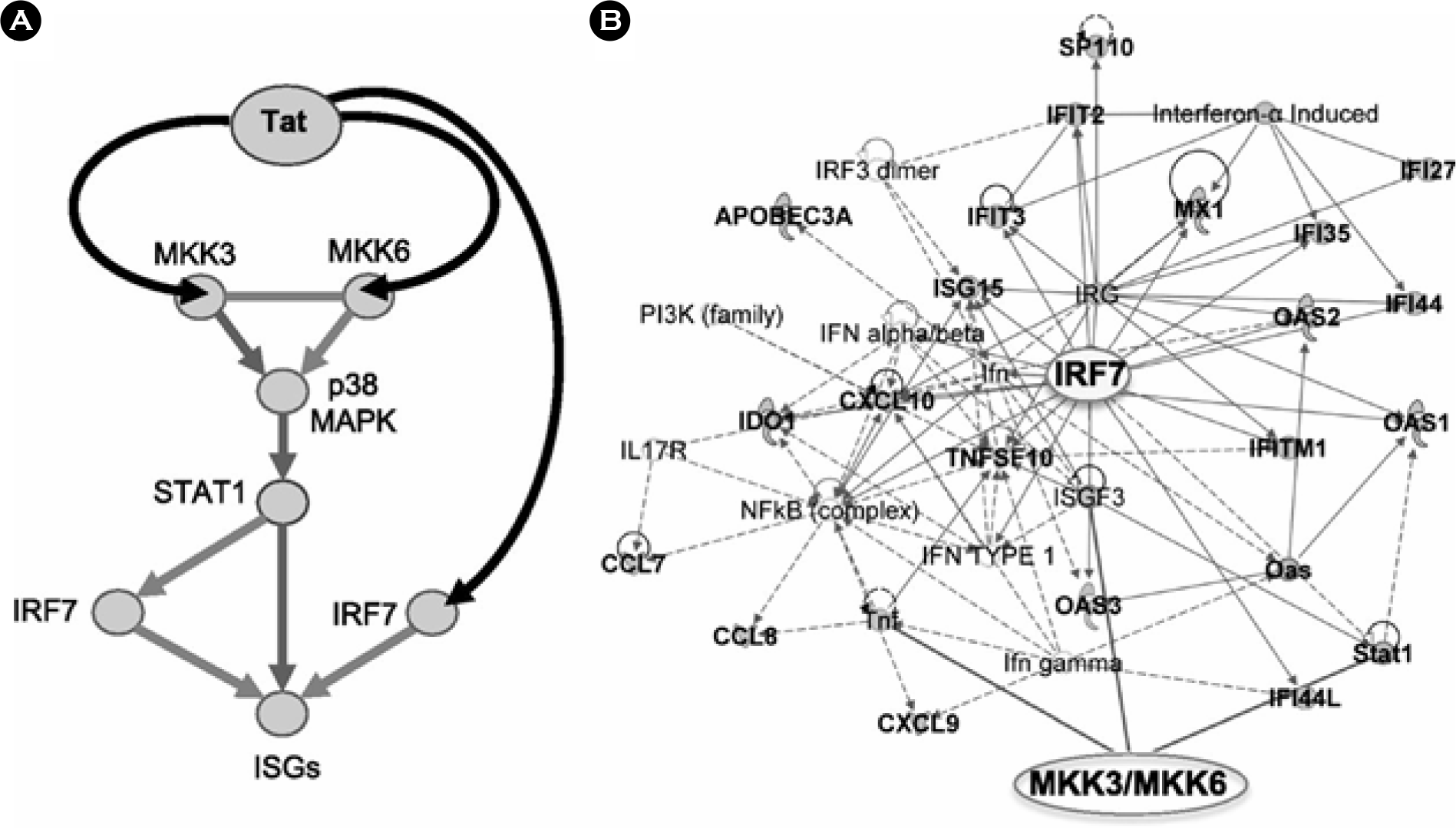 | Figure 4.Schematic of the pathway triggered by Tat and leading to activation of ISGs by Tat in APCs infected by HIV. (A) Tat triggers signaling pathways that activate ISGs by associating with MAPK kinases MAP2K3 (MKK3) and MAP2K6 (MKK6), which in turn activate p38 MAPK and STAT1, and with IRF7 in APCs infected by HIV. (B) Activation of MKK3, MKK6, and IRF7 leads to expression of many ISGs (shown here in connection with MKK3, MKK6, and IRF7), whose product can positively impact T-cell immunoactivation and negatively impact virus production. Bold labeling indicates genes found upregulated by HIV and Tat in APC. Adapted from “Tat engagement of p38 MAP kinase and IRF7 pathways leads to activation of interferon-stimulated genes in antigen-presenting cells”, by Kim et al., 2013, Blood, 121, 4090 as a reference 53 in the text. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download