Abstract
Obesity has been considered a risk factor for infectious diseases including the influenza virus. Most epidemiological investigations indicated that obesity is connected to the severity of influenza, although there are some exceptions. Many studies using obese humans and animal models showed that immune response was impaired in the obese group, increasing susceptibility and severity of influenza virus. However, the exact mechanism by which obesity inhibits anti-viral immune response remains unknown. This review discusses current studies about the properties of immune cells in obesity. In obesity, the balance of adipokines is disrupted and the level of proinflammatory cytokine is increased compared with non-obese control. Moreover, macrophages induced systemic inflammation by secreting cytokines such as TNF-α and IL-6, antigen presenting capacity of dendritic cells was diminished which affect T cell responses, and influenza-specific antibody production seems reduced and decreased even faster after vaccination in obese mouse. The number of circulating T cells and proliferation of mitogen-stimulated T cells dropped and T cell memory was significantly low in influenza infected obese mouse. Therefore, obesity may be one of factors for disease progression in influenza virus infection and vaccine efficacy.
REFERENCES
1). Kim CO, Nam CM, Lee DC, Chang J, Lee JW. Is abdominal obesity associated with the 2009 influenza A (H1N1) pandemic in Korean school-aged children? Influenza Other Respir Viruses. 2012; 6:313–7.

2). Fezeu L, Julia C, Henegar A, Bitu J, Hu FB, Grobbee DE, et al. Obesity is associated with higher risk of intensive care unit admission and death in influenza A (H1N1) patients: a systematic review and meta-analysis. Obes Rev. 2011; 12:653–9.

3). Louie JK, Acosta M, Samuel MC, Schechter R, Vugia DJ, Harriman K, et al. A novel risk factor for a novel virus: obesity and 2009 pandemic influenza A (H1N1). Clin Infect Dis. 2011; 52:301–12.

4). Chippirraz EL, Sorlí L, Montero M, Mas V, Granados EL, Vilaplana C, et al. [Predictive factors for pneumonia in adults infected with the new pandemic A (H1H1) influenza virus]. Rev Esp Quimioter. 2011; 24:204–8.
5). Kwong JC, Campitelli MA, Rosella LC. Obesity and respiratory hospitalizations during influenza seasons in Ontario, Canada: a cohort study. Clin Infect Dis. 2011; 53:413–21.

6). Morgan OW, Bramley A, Fowlkes A, Freedman DS, Taylor TH, Gargiullo P, et al. Morbid obesity as a risk factor for hospitalization and death due to 2009 pandemic influenza A(H1N1) disease. PloS One. 2010; 5:e9694.

7). Coleman LA, Waring SC, Irving SA, Vandermause M, Shay DK, Belongia EA. Evaluation of obesity as an independent risk factor for medically attended laboratory-confirmed influenza. Influenza Other Respir Viruses. 2013; 7:160–7.

8). Blumentals WA, Nevitt A, Peng MM, Toovey S. Body mass index and the incidence of influenza-associated pneumonia in a UK primary care cohort. Influenza Other Respir Viruses. 2012; 6:28–36.

9). Díaz E, Rodríguez A, Martin-Loeches I, Lorente L, del Mar Martín M, Pozo JC, et al. Impact of obesity in patients infected with 2009 influenza A(H1N1). Chest. 2011; 139:382–6.

11). Sheridan PA, Paich HA, Handy J, Karlsson EA, Hudgens MG, Sammon AB, et al. Obesity is associated with impaired immune response to influenza vaccination in humans. Int J Obes (Lond). 2012; 36:1072–7.

12). Milner JJ, Beck MA. The impact of obesity on the immune response to infection. Proc Nutr Soc. 2012; 71:298–306.

13). Esposito S, Preti V, Consolo S, Nazzari E, Principi N. Adenovirus 36 infection and obesity. J Clin Virol. 2012; 55:95–100.

14). Na HN, Nam JH. Infectobesity: a New Area for Microbiological and Virological Research. J Bacteriol Virol. 2011; 41:65–76.

15). Park S, Nam JH. Novel Role of Invariant Natural Killer T-cell in Glycemic Control: Regulation by human Adenovirus 36. J Bacteriol Virol. 2013; 43:229–32.

16). Kim YH, Kim JK, Kim DJ, Nam JH, Shim SM, Choi YK, et al. Diet-induced obesity dramatically reduces the efficacy of a 2009 pandemic H1N1 vaccine in a mouse model. J Infect Dis. 2012; 205:244–51.

17). Yoshihiro K. Influenza Virology. Current Topics. Norfolk:. Caister Academic Press;2006.
18). Hay AJ, Gregory V, Douglas AR, Lin YP. The evolution of human influenza viruses. Philos Trans R Soc Lond B Biol Sci. 2001; 356:1861–70.

19). Matsuzaki Y, Sugawara K, Mizuta K, Tsuchiya E, Muraki Y, Hongo S, et al. Antigenic and genetic characterization of influenza C viruses which caused two outbreaks in Yamagata City, Japan, in 1996 and 1998. J Clin Microbiol. 2002; 40:422–9.

20). Sobrino TCMaF. Animal Viruses: Molecular Biology. Norfolk:. Caister Academic Press;2008.
21). Suzuki Y. Sialobiology of influenza: molecular mechanism of host range variation of influenza viruses. Biol Pharm Bull. 2005; 28:399–408.

22). Hilleman MR. Realities and enigmas of human viral influenza: pathogenesis, epidemiology and control. Vaccine. 2002; 20:3068–87.

23). Parrish CR, Kawaoka Y. The origins of new pandemic viruses: the acquisition of new host ranges by canine parvovirus and influenza A viruses. Annu Rev Microbiol. 2005; 59:553–86.

24). Ballinger MN, Standiford TJ. Postinfluenza bacterial pneumonia: host defenses gone awry. J Interferon Cytokine Res. 2010; 30:643–52.

25). Schmitz N, Kurrer M, Bachmann MF, Kopf M. Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J Virol. 2005; 79:6441–8.

26). Lee I, Kim JI, Park MS. Cell Culture-based Influenza Vaccines as Alternatives to Egg-based Vaccines. J Bacteriol Virol. 2013; 43:9–17.

27). Vaccine use World Health Organization. 2012. [6 December].
28). Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006; 444:847–53.

30). Jo J, Gavrilova O, Pack S, Jou W, Mullen S, Sumner AE, et al. Hypertrophy and/or Hyperplasia: Dynamics of Adipose Tissue Growth. PLoS Comput Biol. 2009; 5:e1000324.

31). Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993; 259:87–91.
32). Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003; 112:1796–808.

33). Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995; 95:2409–15.

34). Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006; 116:1793–801.

35). Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005; 96:939–49.

36). Ouchi N, Walsh K. A novel role for adiponectin in the regulation of inflammation. Arterioscler Thromb Vasc Biol. 2008; 28:1219–21.

37). Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003; 112:1821–30.

38). Fresno M, Alvarez R, Cuesta N. Toll-like receptors, inflammation, metabolism and obesity. Arch Physiol Biochem. 2011; 117:151–64.

39). Mantzoros CS. The role of leptin in human obesity and disease: a review of current evidence. Ann Intern Med. 1999; 130:671–80.

40). Karmazyn M, Purdham DM, Rajapurohitam V, Zeidan A. Signalling mechanisms underlying the metabolic and other effects of adipokines on the heart. Cardiovasc Res. 2008; 79:279–86.

41). Papathanassoglou E, El-Haschimi K, Li XC, Matarese G, Strom T, Mantzoros C. Leptin receptor expression and signaling in lymphocytes: kinetics during lymphocyte activation, role in lymphocyte survival, and response to high fat diet in mice. J Immunol. 2006; 176:7745–52.

42). Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol. 2000; 68:437–46.
43). Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998; 394:897–901.

44). Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, et al. Leptin regulates proinflammatory immune responses. FASEB J. 1998; 12:57–65.

45). Mattioli B, Straface E, Quaranta MG, Giordani L, Viora M. Leptin promotes differentiation and survival of human dendritic cells and licenses them for Th1 priming. J Immunol. 2005; 174:6820–8.

46). Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum Immunoreactive-Leptin Concentrations in Normal-Weight and Obese Humans. N Engl J Med. 1996; 334:292–5.

47). Faggioni R, Jones-Carson J, Reed DA, Dinarello CA, Feingold KR, Grunfeld C, et al. Leptin-deficient (ob/ob) mice are protected from T cell-mediated hepatotoxicity: role of tumor necrosis factor alpha and IL-18. Proc Natl Acad Sci U S A. 2000; 97:2367–72.

48). Conge GA, Gouache P, Joyeux Y, Goichot J, Fournier JM. [Influence of different types of experimental obesity on resistance of the mouse to infection by Salmonella typhimurium and Klebsiella pneumoniae]. Ann Nutr Metab. 1988; 32:113–20.
49). Chandra RK, Au B. Spleen hemolytic plaque-forming cell response and generation of cytotoxic cells in genetically obese (C57Bl/6J ob/ob) mice. Int Arch Allergy Appl Immunol. 1980; 62:94–8.
50). Mandel MA, Mahmoud AA. Impairment of cell-mediated immunity in mutation diabetic mice (db/db). J Immunol. 1978; 120:1375–7.
51). Faggioni R, Fantuzzi G, Gabay C, Moser A, Dinarello CA, Feingold KR, et al. Leptin deficiency enhances sensitivity to endotoxin-induced lethality. Am J Physiol. 1999; 276:R136–42.

52). Yang SQ, Lin HZ, Lane MD, Clemens M, Diehl AM. Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proc Natl Acad Sci U S A. 1997; 94:2557–62.

53). Charles A, Janeway J, Travers Paul, Walport Mark, Shlomchik Mark J. Immunobiology. 5th ed.Garland Science;2001.
54). Mills CD. M1 and M2 Macrophages: Oracles of Health and Disease. Crit Rev Immunol. 2012; 32:463–88.

55). Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007; 117:175–84.

56). Plotkin BJ, Paulson D. Zucker rat (fa/fa), a model for the study of immune function in type-II diabetes mellitus: effect of exercise and caloric restriction on the phagocytic activity of macrophages. Lab Anim Sci. 1996; 46:682–4.
57). Karlsson EA, Beck MA. The burden of obesity on infectious disease. Exp Biol Med (Maywood). 2010; 235:1412–24.

58). Gurney KB, Colantonio AD, Blom B, Spits H, Uittenbogaart CH. Endogenous IFN-alpha production by plasmacytoid dendritic cells exerts an antiviral effect on thymic HIV-1 infection. J Immunol. 2004; 173:7269–76.
59). Kaliński P, Hilkens CM, Snijders A, Snijdewint FG, Kapsenberg ML. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J Immunol. 1997; 159:28–35.
60). Trinchieri G. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv Immunol. 1998; 70:83–243.

61). Macia L, Delacre M, Abboud G, Ouk TS, Delanoye A, Verwaerde C, et al. Impairment of dendritic cell functionality and steady-state number in obese mice. J Immunol. 2006; 177:5997–6006.

62). Smith AG, Sheridan PA, Tseng RJ, Sheridan JF, Beck MA. Selective impairment in dendritic cell function and altered antigen-specific CD8+ T-cell responses in diet-induced obese mice infected with influenza virus. Immunology. 2009; 126:268–79.
63). Karlsson EA, Sheridan PA, Beck MA. Diet-induced obesity impairs the T cell memory response to influenza virus infection. J Immunol. 2010; 184:3127–33.

64). Suárez-Álvarez K, Solís-Lozano L, Leon-Cabrera S, González-Chávez A, Gómez-Hernández G, Quiñones-Álvarez MS, et al. Serum IL-12 is increased in Mexican obese subjects and associated with low-grade inflammation and obesity-related parameters. Mediators Inflamm. 2013; 2013:967067.

66). MacLennan IC, Toellner KM, Cunningham AF, Serre K, Sze DM, Zúñiga E, et al. Extrafollicular antibody responses. Immunol Rev. 2003; 194:8–18.

67). Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral Immunity Due to Long-Lived Plasma Cells. Immunity. 1998; 8:363–72.

68). Tangye SG, Avery DT, Deenick EK, Hodgkin PD. Intrinsic differences in the proliferation of naive and memory human B cells as a mechanism for enhanced secondary immune responses. J Immunol. 2003; 170:686–94.

69). Tangye SG, Tarlinton DM. Memory B cells: effectors of long-lived immune responses. Eur J Immunol. 2009; 39:2065–75.

70). Kelly DF, Pollard AJ, Moxon ER. Immunological memory: the role of B cells in long-term protection against invasive bacterial pathogens. JAMA. 2005; 294:3019–23.
71). Zhang X, Wang Q, Bi Y, Kou Z, Zhou J, Cui Y, et al. Kinetics of Memory B Cell and Plasma Cell Responses in the Mice Immunized with Plague Vaccines. Scand J Immunol. 2014; 79:157–62.

72). Smith AG, Sheridan PA, Harp JB, Beck MA. Diet-induced obese mice have increased mortality and altered immune responses when infected with influenza virus. J Nutr. 2007; 137:1236–43.

73). Park HL, Shim SH, Lee EY, Cho W, Park S, Jeon HJ, et al. Obesity-induced chronic inflammation is associated with the reduced efficacy of influenza vaccine. Hum Vaccin Immunother. 2014; 10.

74). Pozzi LA, Maciaszek JW, Rock KL. Both dendritic cells and macrophages can stimulate naive CD8 T cells in vivo to proliferate, develop effector function, and differentiate into memory cells. J Immunol. 2005; 175:2071–81.
75). Graham MB, Braciale TJ. Resistance to and recovery from lethal influenza virus infection in B lymphocyte-deficient mice. J Exp Med. 1997; 186:2063–8.

76). Belz GT, Wodarz D, Diaz G, Nowak MA, Doherty PC. Compromised influenza virus-specific CD8 (+)-T-cell memory in CD4 (+)-T-cell-deficient mice. J Virol. 2002; 76:2388–93.
77). Kreijtz JH, de Mutsert G, van Baalen CA, Fouchier RA, Osterhaus AD, Rimmelzwaan GF. Cross-recognition of avian H5N1 influenza virus by human cytotoxic T-lymphocyte populations directed to human influenza A virus. J Virol. 2008; 82:5161–6.

78). Lee LY, Ha do LA, Simmons C, de Jong MD, Chau NV, Schumacher R, et al. Memory T cells established by seasonal human influenza A infection cross-react with avian influenza A (H5N1) in healthy individuals. J Clin Invest. 2008; 118:3478–90.

79). O'Rourke RW, Kay T, Scholz MH, Diggs B, Jobe BA, Lewinsohn DM, et al. Alterations in T-cell subset frequency in peripheral blood in obesity. Obes Surg. 2005; 15:1463–8.
80). Tanaka S, Isoda F, Ishihara Y, Kimura M, Yamakawa T. T lymphopaenia in relation to body mass index and TNF-alpha in human obesity: adequate weight reduction can be corrective. Clin Endocrinol (Oxf). 2001; 54:347–54.

81). Tanaka S, Inoue S, Isoda F, Waseda M, Ishihara M, Yamakawa T, et al. Impaired immunity in obesity: suppressed but reversible lymphocyte responsiveness. Int J Obes Relat Metab Disord. 1993; 17:631–6.
82). Kimura M, Tanaka S, Isoda F, Sekigawa K, Yamakawa T, Sekihara H. T lymphopenia in obese diabetic (db/db) mice is non-selective and thymus independent. Life Sci. 1998; 62:1243–50.
83). Tanaka Si, Isoda F, Yamakawa T, Ishihara M, Sekihara H. T lymphopenia in genetically obese rats. Clin Immunol Immunopathol. 1998; 86:219–25.

84). Sato Mito N, Suzui M, Yoshino H, Kaburagi T, Sato K. Long term effects of high fat and sucrose diets on obesity and lymphocyte proliferation in mice. J Nutr Health Aging. 2009; 13:602–6.

Figure 1.
Obesity induces inflammation, leptin resistance, and immune defect. Obesity-related inflammation gives rise to leptin resistance thereby immune cells do not normally respond to immunogens such as virus and vaccine.
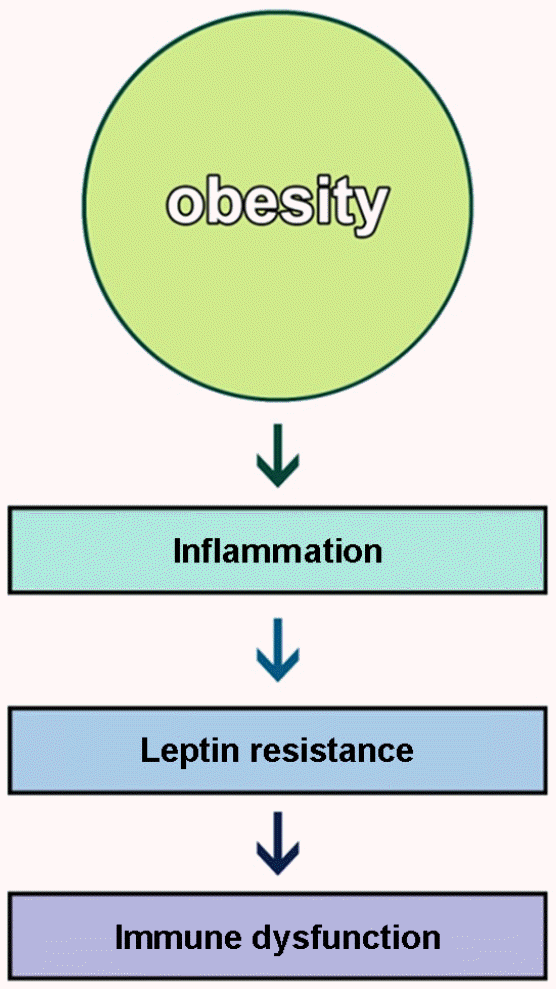
Figure 2.
Impacts of obesity on immune cells. Obesity induces infiltration and M1 polarization of macrophage which produces large amount of inflammatory cytokines. The number of DCs is decreased in obesity and obese DCs fail to induce T cell response due to reduced antigen presentation and IL-12 production. Antibody production by obese B cells is lower than those of lean controls. In T cells, the number of circulating T cells, proliferation, cytokine production, memory response, and CD4 T cell activation in response to stimuli are reduced in obesity.
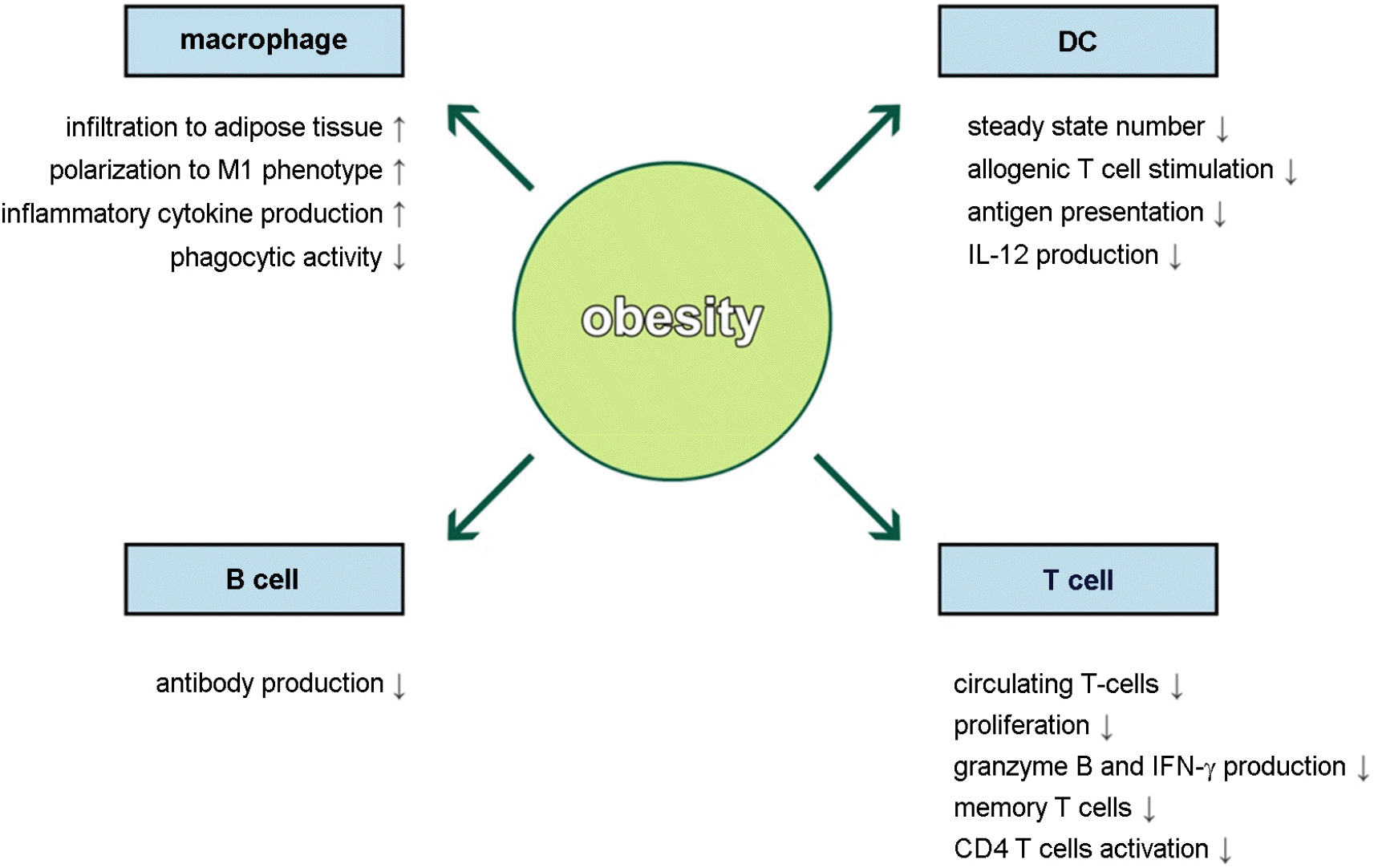
Table 1.
Human studies for relationship between obesity and influenza infection




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download