Abstract
Angiostatin is derived from enzymatic degradation of plasminogen and it has endogenous anti-angiogenic properties. Although tumor cells, macrophages, platelets, and neutrophils generate high amount of angiostatin, its expression is increased in inflammatory conditions. Moreover, angiostatin binds to integrin αvβ3, ATP synthase, and angiomotin, which expressed on neutrophils. Activated neutrophils are essential to innate immune response, but also cause tissue damage through production of reactive oxygen species (ROS) and increase lifespan. In this article, it suggests several mechanism of angiostatin as immune regulator for neutrophils in inflammatory conditions; complex with integrin αvβ3 and F1F0 ATP synthase on lipid raft, attenuate polarization, and ROS production. These data provide possible exploit of double-edged role of neutrophils in acute inflammatory pathologies to preserve beneficial effect and minimize tissue damage.
REFERENCES
1). Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012; 30:459–89.

2). Fialkow L, Wang Y, Downey GP. Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radic Biol Med. 2007; 42:153–64.

4). Insall RH. Understanding eukaryotic chemotaxis: a pseudopod-centred view. Nat Rev Mol Cell Biol. 2010; 11:453–8.

5). Niggli V. Signaling to migration in neutrophils: importance of localized pathways. Int J Biochem Cell Biol. 2003; 35:1619–38.

6). Khan AI, Heit B, Andonegui G, Colarusso P, Kubes P. Lipopolysaccharide: a p38 MAPK-dependent disrupter of neutrophil chemotaxis. Microcirculation. 2005; 12:421–32.

7). Hino M, Kurogi K, Okubo MA, Murata-Hori M, Hosoya H. Small heat shock protein 27 (HSP27) associates with tubulin/microtubules in HeLa cells. Biochem Biophys Res Commun. 2000; 271:164–9.

8). Jog NR, Jala VR, Ward RA, Rane MJ, Haribabu B, McLeish KR. Heat shock protein 27 regulates neutrophil chemotaxis and exocytosis through two independent mechanisms. J Immunol. 2007; 178:2421–8.

9). Barreiro O, de la Fuente H, Mittelbrunn M, Sánchez-Madrid F. Functional insights on the polarized redistribution of leukocyte integrins and their ligands during leukocyte migration and immune interactions. Immunol Rev. 2007; 218:147–64.

10). Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007; 7:678–89.

11). Lawson MA, Maxfield FR. Ca(2+)- and calcineurin-dependent recycling of an integrin to the front of migrating neutrophils. Nature. 1995; 377:75–9.

12). Moon C, Han JR, Park HJ, Hah JS, Kang JL. Synthetic RGDS peptide attenuates lipopolysaccharide-induced pulmonary inflammation by inhibiting integrin signaled MAP kinase pathways. Respir Res. 2009; 10:18.

13). Rainger GE, Buckley CD, Simmons DL, Nash GB. Neutrophils sense flow-generated stress and direct their migration through alphaVbeta3-integrin. Am J Physiol. 1999; 276:H858–64.
14). Singh B, Janardhan KS, Kanthan R. Expression of angiostatin, integrin alphavbeta3, and vitronectin in human lungs in sepsis. Exp Lung Res. 2005; 31:771–82.
15). Jurasz P, Santos-Martinez MJ, Radomska A, Radomski MW. Generation of platelet angiostatin mediated by urokinase plasminogen activator: effects on angiogenesis. J Thromb Haemost. 2006; 4:1095–106.

16). O'Mahony CA, Seidel A, Albo D, Chang H, Tuszynski GP, Berger DH. Angiostatin generation by human pancreatic cancer. J Surg Res. 1998; 77:55–8.
17). Scapini P, Nesi L, Morini M, Tanghetti E, Belleri M, Noonan D, et al. Generation of biologically active angiostatin kringle 1–3 by activated human neutrophils. J Immunol. 2002; 168:5798–804.
18). Westphal JR, Van't Hullenaar R, Geurts-Moespot A, Sweep FC, Verheijen JH, Bussemakers MM, et al. Angiostatin generation by human tumor cell lines: involvement of plasminogen activators. Int J Cancer. 2000; 86:760–7.

19). Wahl ML, Kenan DJ, Gonzalez-Gronow M, Pizzo SV. Angiostatin's molecular mechanism: aspects of specificity and regulation elucidated. J Cell Biochem. 2005; 96:242–61.

20). Lee TY, Muschal S, Pravda EA, Folkman J, Abdollahi A, Javaherian K. Angiostatin regulates the expression of antiangiogenic and proapoptotic pathways via targeted inhibition of mitochondrial proteins. Blood. 2009; 114:1987–98.

21). Dudani AK, Mehic J, Martyres A. Plasminogen and angiostatin interact with heat shock proteins. Mol Cell Biochem. 2007; 300:197–205.

22). Sharma MR, Rothman V, Tuszynski GP, Sharma MC. Antibody-directed targeting of angiostatin's receptor annexin II inhibits Lewis Lung Carcinoma tumor growth via blocking of plasminogen activation: possible biochemical mechanism of angiostatin's action. Exp Mol Pathol. 2006; 81:136–45.

23). Tarui T, Miles LA, Takada Y. Specific interaction of angiostatin with integrin alpha(v)beta(3) in endothelial cells. J Biol Chem. 2001; 276:39562–8.
24). Troyanovsky B, Levchenko T, Månsson G, Matvijenko O, Holmgren L. Angiomotin: an angiostatin binding protein that regulates endothelial cell migration and tube formation. J Cell Biol. 2001; 152:1247–54.
25). Hamacher J, Lucas R, Lijnen HR, Buschke S, Dunant Y, Wendel A, et al. Tumor necrosis factor-alpha and angiostatin are mediators of endothelial cytotoxicity in bronchoalveolar lavages of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2002; 166:651–6.
26). Luca R, Lijnen HR, Suffredini AF, Pepper MS, Steinberg KP, Martin TR, et al. Increased angiostatin levels in bronchoalveolar lavage fluids from ARDS patients and from human volunteers after lung instillation of endotoxin. Thromb Haemost. 2002; 87:966–71.

27). Benelli R, Morini M, Carrozzino F, Ferrari N, Minghelli S, Santi L, et al. Neutrophils as a key cellular target for angiostatin: implications for regulation of angiogenesis and inflammation. FASEB J. 2002; 16:267–9.

28). Chavakis T, Athanasopoulos A, Rhee JS, Orlova V, Schmidt-Wöll T, Bierhaus A, et al. Angiostatin is a novel anti-inflammatory factor by inhibiting leukocyte recruitment. Blood. 2005; 105:1036–43.

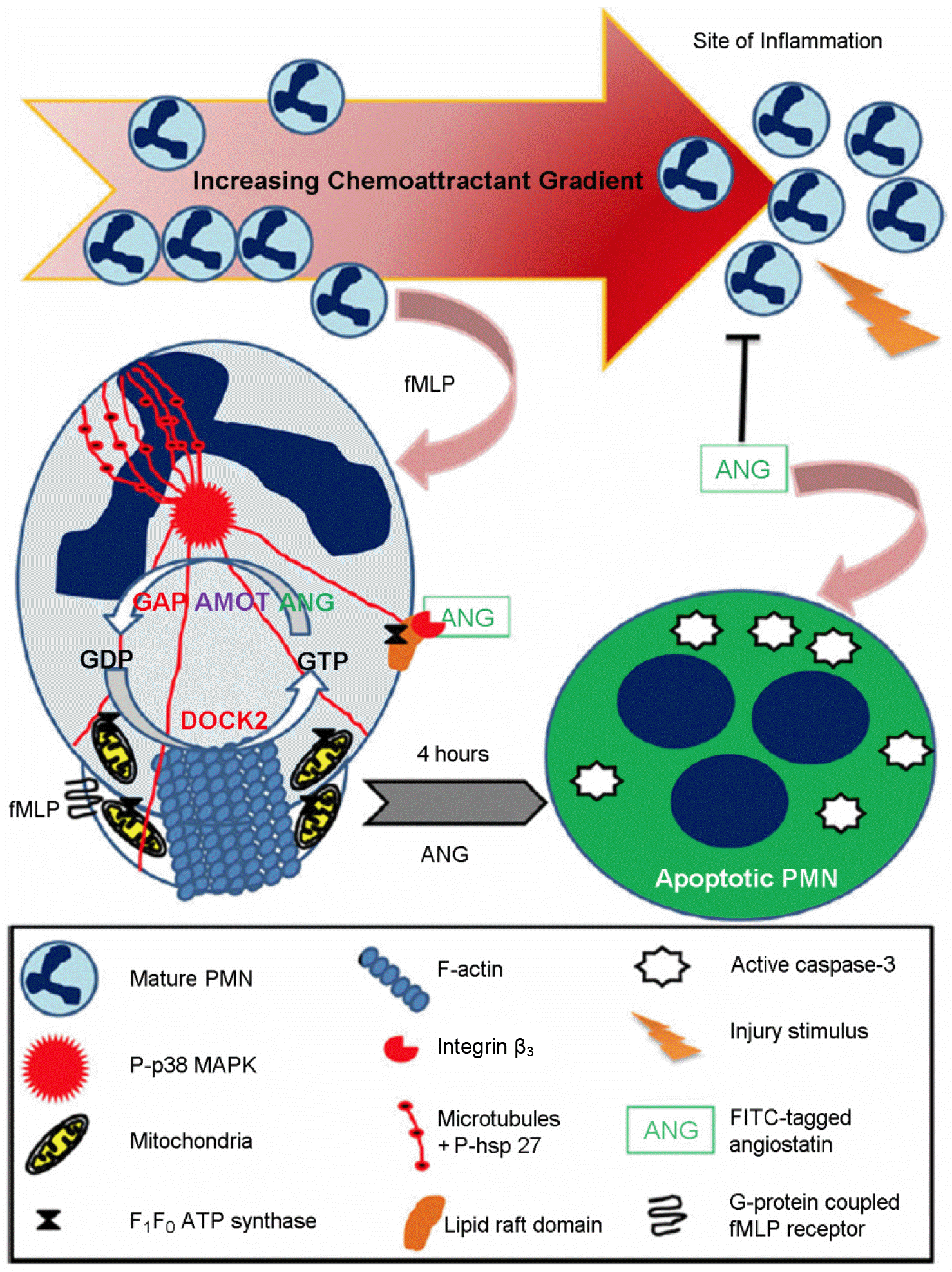
Figure 1.
Schematic diagram for mechanism of action of angiostatin (ANG) in acute inflammation (30). ANG inhibits neutrophil adhesion as well as trans-endothelial migration in an acute inflammation setting by inhibiting the leading edge actin dynamics. Angiostatin is endocytosed in activated neutrophils via lipid raft domains, which express integrin αvβ3 as well as F1F0 ATP synthase. It can be hypothesized that ANG binds to a GTPase activating protein (GAP) associated angiomotin (AMOT) that inhibits guanosine triphophate (GTP) recycling required for F-actin aggregation at the leading edge, thereby inhibiting neutrophil chemotaxis. Decrease of the mitochondrial ATP synthesis via inhibition of F1F0 ATP synthase leads to mitochondrial redox inhibition and reduction in ROS production and ultimately induces apoptosis, as evidenced through the presence of apoptotic nuclear bodies (depicted in blue) after 4 hours of angiostatin incubation with LPS (30).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download