Abstract
HIV-1 Tat protein has been implicated as a causative agent in the pathogenesis of HIV-1-associated neurocognitive disorder (HAND) and Alzheimer's disease (AD)-like pathology in HIV-1 infected patients. Here, we provide insights into the potential roles of extracellular HIV-1 Tat protein in amyloid β (Aβ) generation and Tau phosphorylation, two major neuropathological features of AD. Exposure of the rat hippocampal slices to the full-length HIV-1 Tat protein (Tat1-86) for 3 days led to the increased levels of Aβ precursor protein (APP) accumulation, which accompanied by Aβ generation in the hippocampus, the brain region most commonly damaged in HIV-1-associated dementia (HAD). Moreover, extracellular HIV-1 Tat significantly stimulated the level of phosphrylated Tau (pTau) identified using immunoblotting with AT8 antibody, which recognizes abnormally hyperphosphorylated Tau. Collectively, our data suggest that HIV-1 Tat plays important roles in increasing the levels of APP accumulation, Aβ generation and Tau phosphorylation in the hippocampus, and thereby might contribute to the development of AD-like pathology in HIV-1-infected patients.
Go to : 
REFERENCES
1). Gannon P, Khan MZ, Kolson DL. Current understanding of HIV-associated neurocognitive disorders pathogenesis. Curr Opin Neurol. 2011; 24:275–83.

2). Irish BP, Khan ZK, Jain P, Nonnemacher MR, Pirrone V, Rahman S, et al. Molecular mechanisms of neuro-degenerative diseases induced by human retroviruses: a review. Am J Infect Dis. 2009; 5:231–58.

3). Heaton RK, Clifford DB, Franklin DR Jr, Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010; 75:2087–96.

4). Harezlak J, Buchthal S, Taylor M, Schifitto G, Zhong J, Daar E, et al. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS. 2011; 25:625–33.

5). Clifford DB, Fagan AM, Holtzman DM, Morris JC, Teshome M, Shah AR, et al. CSF biomarkers of Alzheimer disease in HIV-associated neurologic disease. Neurology. 2009; 73:1982–7.

6). Xu J, Ikezu T. The comorbidity of HIV-associated neurocognitive disorders and Alzheimer's disease: a foreseeable medical challenge in post-HAART era. J Neuroimmune Pharmacol. 2009; 4:200–12.

7). Lukiw WJ. Amyloid beta (Aβ) peptide modulators and other current treatment strategies for Alzheimer's disease (AD). Expert Opin Emerg Drugs. 2012; 17:43–60.

8). Jucker M, Walker LC. Pathogenic protein seeding in Alzheimer disease and other neurodegenerative disorders. Ann Neurol. 2011; 70:532–40.

9). Zilka N, Kazmerova Z, Jadhav S, Neradil P, Madari A, Obetkova D, et al. Who fans the flames of Alzheimer's disease brains? Misfolded tau on the crossroad of neurodegenerative and inflammatory pathways. J Neuroinflammation. 2012; 9:47.

10). Romani B, Engelbrecht S, Glashoff RH. Functions of Tat: the versatile protein of human immunodeficiency virus type 1. J Gen Virol. 2010; 91:1–12.

11). Banks WA, Robinson SM, Nath A. Permeability of the blood-brain barrier to HIV-1 Tat. Exp Neurol. 2005; 193:218–27.

12). Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis. 2002; 186:S193–8.

13). Green DA, Masliah E, Vinters HV, Beizai P, Moore DJ, Achim CL. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS. 2005; 19:407–11.

14). Giometto B, An SF, Groves M, Scaravilli T, Geddes JF, Miller R, et al. Accumulation of beta-amyloid precursor protein in HIV encephalitis: relationship with neuropsychological abnormalities. Ann Neurol. 1997; 42:34–40.
15). Andersson L, Blennow K, Fuchs D, Svennerholm B, Gisslé M. Increased cerebrospinal fluid protein tau concentration in neuro-AIDS. J Neurol Sci. 1999; 171:92–6.

16). Brew BJ, Pemberton L, Blennow K, Wallin A, Hagberg L. CSF amyloid beta42 and tau levels correlate with AIDS dementia complex. Neurology. 2005; 65:1490–2.
17). Chong YH, Shin YJ, Lee EO, Kayed R, Glabe CG, Tenner AJ. ERK1/2 activation mediates Abeta oligomer-induced neurotoxicity via caspase-3 activation and tau cleavage in rat organotypic hippocampal slice cultures. J Biol Chem. 2006; 281:20315–25.
18). Lee EO, Kim SE, Park HK, Kang JL, Chong YH. Extracellular HIV-1 Tat upregulates TNF-α dependent MCP-1/CCL2 production via activation of ERK1/2 pathway in rat hippocampal slice cultures: Inhibition by resveratrol, a polyphenolic phytostilbene. Exp Neurol. 2011; 229:399–408.

19). Holopainen IE. Organotypic hippocampal slice cultures: a model system to study basic cellular and molecular mechanisms of neuronal cell death, neuroprotection, and synaptic plasticity. Neurochem Res. 2005; 30:1521–8.

20). Agrawal L, Louboutin JP, Reyes BA, Van Bockstaele EJ, Strayer DS. HIV-1 Tat neurotoxicity: a model of acute and chronic exposure, and neuroprotection by gene delivery of antioxidant enzymes. Neurobiol Dis. 2012; 45:657–70.

21). Buscemi L, Ramonet D, Geiger JD. Human immunodeficiency virus type-1 protein Tat induces tumor necrosis factor-alpha-mediated neurotoxicity. Neurobiol Dis. 2007; 26:661–70.
22). Giunta B, Hou H, Zhu Y, Rrapo E, Tian J, Takashi M, et al. HIV-1 Tat contributes to Alzheimer's disease-like pathology in PSAPP mice. Int J Clin Exp Pathol. 2009; 2:433–43.
23). Rempel HC, Pulliam L. HIV-1 Tat inhibits neprilysin and elevates amyloid beta. AIDS. 2005; 19:127–35.
24). Aksenov MY, Aksenova MV, Nath A, Ray PD, Mactutus CF, Booze RM. Cocaine-mediated enhancement of Tat toxicity in rat hippocampal cell cultures: the role of oxidative stress and D1 dopamine receptor. Neurotoxicology. 2006; 27:217–28.

25). Aksenova MV, Aksenov MY, Adams SM, Mactutus CF, Booze RM. Neuronal survival and resistance to HIV-1 Tat toxicity in the primary culture of rat fetal neurons. Exp Neurol. 2009; 215:253–63.

26). Kruman II, Nath A, Mattson MP. HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp Neurol. 1998; 154:276–88.

27). Lee EO, Yang JH, Kim HJ, Woo SY, Chong YH. HIV-1 Tat protein-dependent cytotoxicity is attenated by 15-deoxy-Delta12,14-prostaglandin J2 in rat hippocampal slices: involvement of the ERK1/2 signaling pathway. J Bacteriol Virol. 2013; 43:45–53.
28). Giunta B, Zhou Y, Hou H, Rrapo E, Fernandez F, Tan J. HIV-1 TAT inhibits microglial phagocytosis of Abeta peptide. Int J Clin Exp Pathol. 2008; 1:260–75.
Go to : 
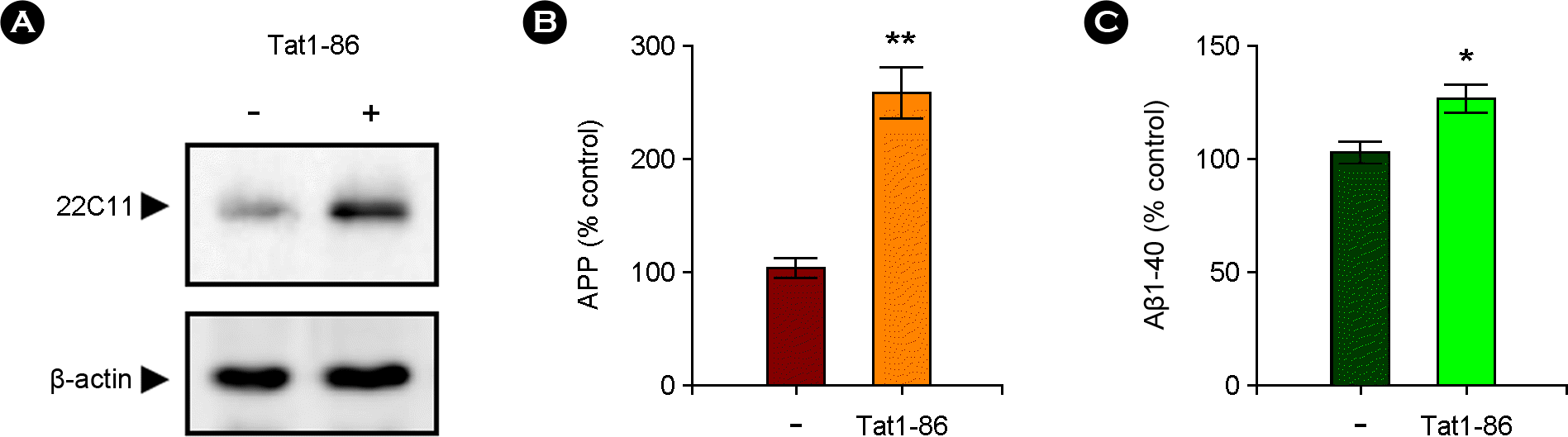 | Figure 1.Tat1-86 increased the level of APP accumulation and Aβ1-40 generation in the hippocampus. The hippocampal slices were treated for 3 days with Tat1-86 (1 μM) or vehicle only. (A) Western blots showing the Tat-induced increase of APP accumulation in hippocampal slices. Total lysates were analyzed via immunoblotting with 22C11 antibody to identify membrane APP or soluble form of APP derivatives. The blots were stripped and developed with anti-β-actin for equal protein loading. The data represent three independent experiments. (B) A normalized densitometric quantification of APP against β-actin for equal protein loading. (C) The levels of Aβ1-40 released in the slice culture media were assessed by using a specific Aβ ELISA kit. The results of triplicate experiments are expressed as the means ± SEM. *p < 0.05 versus vehicle-treated samples. |
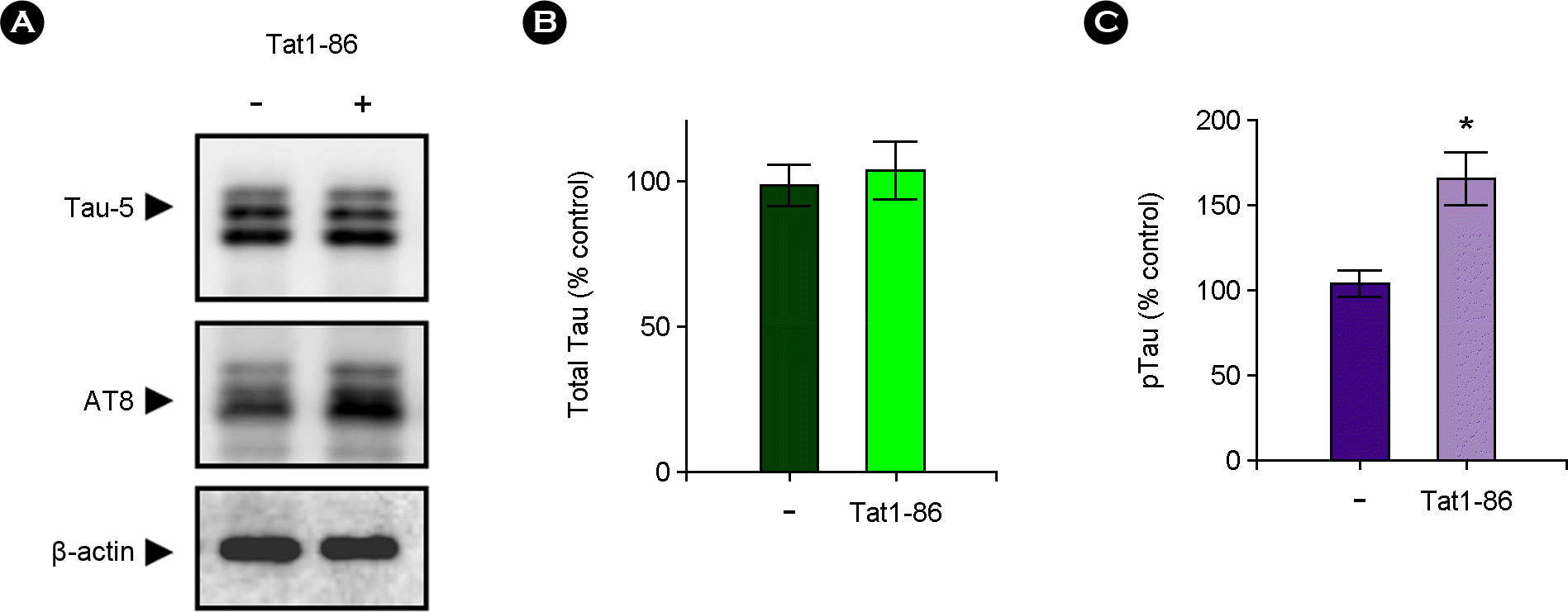 | Figure 2.Tat1-86 promoted the level of phosphorylated Tau (pTau) in hippocampal slices. The slices were treated for 3 days with Tat1-86 (1 μM) or vehicle only. (A) Western blots showing the Tat-induced increase of highly phosphorylated Tau (pTau) in hippocampal slices. Total lysates were analyzed via immunoblotting with Tau-5 antibody to recognize total Tau protein or with AT8 antibody to detect hyperphosphorylated Tau. (B) A normalized densitometric quantification of total Tau against β-actin for equal protein loading. (C) A normalized densitometric quantification of pTau against β-actin for equal protein loading. The results of triplicate experiments are expressed as the means ± SEM. *p < 0.05 versus vehicle-treated samples. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download