Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) is one of the most important nosocomial pathogens worldwide. This study was performed to investigate the characterization of MRSA isolated from healthy persons in Gwangju area. A total of 404 nasal swab samples was collected during October 2011 and May 2012 in Gwangu, Korea. A survey on MRSA was conducted with meat distributors (n=230), pre-school children (n=108), officers (n=66), respectively. To confirm the MRSA, polymerase chain reaction (PCR) for the S. aureus specific gene and mecA gene was performed. A total of 34 (8.4%) MRSA isolates was isolated from 404 nasal swab samples: 6.1% (14/230) from meat distributors, 16.7% (18/108) from pre-school children, and 3.0% (2/66) from officers samples, respectively. The most prevalent antimicrobial resistance observed in the MRSA isolates was to ampicillin 100% (34/34), followed by penicillin 97.1% (33/34), oxacillin 94.1% (32/34) and erythromycin 52.9% (18/34). All MRSA isolates were then characterized by panton-valentine leukocidin (pvl) gene detected by PCR, staphylococcal cassette chromosome mec (SCCmec) typing, and pulsed-field gel electrophoresis (PFGE) with Sma I digestion. 34 MRSA isolates from nasal carriage were pvl gene negative, SCCmec type IV; 73.5% (25/34), type II; 17.6% (6/34), type III; 2.9% (1/34), and untypable; 5.9% (2/34), respectively. 34 MRSA isolates showed 16 PFGE patterns. These results indicated that isolation rates of community-associated methicillin-resistant S. aureus (CA-MRSA) from healthy persons were low (8.4%), but continuous surveillance and monitoring should be performed to prevent the spread of MRSA in the community.
Go to : 
REFERENCES
1). Timoney JF, Gillespie JH, Scott FW, Barlough JE. Hagan and Bruner's Microbiology and Infectious Disease of Domestic Animals. 8th ed.Ithaca: Comstock Publishing Associates;;1988. p. 171–96.
2). Diederen BM, Kluytmans JA. The emergence of Infections with community-associated methicillin resistant Staphylococcus aureus. J Infect. 2006; 52:157–68.
3). Chong Y, Lee K. Present situation of antimicrobial resistant in Korea. J Infect Chemother. 2000; 6:189–95.
4). Kim HB, Jang HC, Nam HJ, Lee YS, Kim BS, Park WB, et al. In vitro activities of 28 antimicrobial agents against Staphylococcus aureus isolates from tertiary-care hospitals in Korea: a nationwide survey. Antimicrob Agents Chemother. 2004; 48:1124–7.
5). Goetz A, Posey K, Fleming J, Jacobs S, Boody L, Wagener MM, et al. Methicillin-resistant Staphylococcus aureus in the community: a hospital-based study. Infect Control Hosp Epidemiol. 1999; 20:689–91.
6). Kallen AJ, Driscoll TJ, Thornton S, Olson PE, Wallace MR. Increase in community-acquired methicillin-resistant Staphylococcus aureus at a Naval Medical Center. Infect Control Hosp Epidemiol. 2000; 21:223–6.
7). From the Centers for Disease Control and Prevention. Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus-Minnesota and North Dakota, 1997–1999. JAMA. 1999; 282:1123–5.
8). Lee JS, Park O, Woo HJ, Jung HJ, Kim WJ, Kim MJ, et al. A Longitudinal Molecular Epidemiologic Study of Methicillin-resistant Staphylococcus aureus (MRSA) Isolates from a University Hospital. Korean J Infect Dis. 2001; 33:32–9.
9). Chambers HF. Methicillin resistance in Staphylococci: molecular and biochemical basis and clinical implications. Clin Microbiol Rev. 1997; 10:781–91.

10). Boyle-Varva S, Ereshefsky B, Wang CC, Daum RS. Successful multiresistant community-associated methicillin-resistant Staphylococcus aureus lineage from Taipei, Taiwan, that carries either the novel staphylococcal chromosome cassette mec (SCCmec) type VT or SCCmec type IV. J Clin Microbiol. 2005; 43:4719–30.
11). Ito T, Ma XX, Takeuchi F, Okuma K, Yuzawa H, Hiramatsu K. Novel type V staphylococcal cassette chromosome mec driven by a novel cassette chromosome recombinase, ccrC. Antimicrob Agents Chemother. 2004; 48:2637–51.
12). Oliveira DC, de Lencastre H. Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2002; 46:2155–61.
13). Cha EK, Chang KS, Hwang SM. Correlation between staphylococcal cassette chromosome mec type and coagulase serotype of methicillin-resistant Staphylococcus aureus. J Bacteriol Virol. 2009; 39:71–8.
14). Chongtrakool P, Ito T, Ma XX, Kondo Y, Trakulsomboon S, Tiensasitorn C, et al. Staphylococcal cassette chromosome mec (SCCmec) typing of methicillin-resistant Staphylococcus aureus strains isolated in 11 Asian countries: a proposal for a new nomenclature for SCCmec elements. Antimicrob Agents Chemother. 2006; 50:1001–12.
15). Choe YJ, Lee SY, Sung JY, Yang MA, Lee JH, Oh CE, et al. A review of Staphylococcus aureus infections in children with an emphasis on community-associated methicillin-resistant S. aureus infections. Korean J Pediatr Infect Dis. 2009; 16:150–61.
16). Park JY, Kim HO, Jeong YG, Kim S, Bae IG. Clinical characteristics and risk factors of community-acquired methicillin-resistant Staphylococcus aureus infections: comparison of community-acquired methicillin-susceptible Staphylococcus aureus infections. Infect Chemother. 2006; 38:109–15.
17). Mason WJ, Blevins JS, Beenken K, Wibowo N, Ojha N, Smeltzer MS. Multiplex PCR protocol for the diagnosis of staphylococcal infection. J Clin Microbiol. 2001; 39:3332–8.

18). Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966; 45:493–6.

19). Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; Eighteenth information supplement, M100-S18. Wayne, PA: CLSI;2008.
20). Boye K, Bartels MD, Andersen IS, Møller JA, Westh H. A New multiplex PCR for easy screening of methicillin-reistant Staphylococccus aureus SCCmec types I-V. Clin Microbiol Infect. 2007; 13:725–7.
21). Lina G, Piémont Y, Godail-Gamot F, Bes M, Peter MO, Gauduchon V, et al. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis. 1999; 29:1128–32.
22). Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995; 33:2233–9.

23). Kim HB, Shin DH, Park KU, Oh MD, Kim EC, Choe KW. The methicillin-resistance rate of Stapylococccus aureus isolated from anterior nares of healthy adults in the community. Infect Chemother. 1998; 30:527–31.
24). Cho JK, Kim JH, Sung MS, Kim KS. Genetic characterization of methicillin-resistant Staphylococcus aureus from humans and animals within the community. Korean J Vet Res. 2011; 51:267–75.
25). Kwon YI, Kim TW, Kim HY, Chang YH, Kwak HS, Woo GJ, et al. Monitoring of methicillin resistant Staphylococcus aureus from medical environment in Korea. Korean J Microbiol Biotechnol. 2007; 35:158–62.
26). Kim JS, Kim HS, Song W, Cho HC, Lee KM, Kim EC. Antimicrobial resistance profiles of Staphylococcus aureus isolated in 13 Korean hospitals. Korean J Lab Med. 2004; 24:223–9.
27). Lee HW, Yoon JH, Sohn JH, Lee KH, Yeh BI, Park DW, et al. Detection of mecA gene in clinical isolates of Staphylococcus aureus by multiplex-PCR, and antimicrobial susceptibility of MRSA. J Microbiol Biotechnol. 2003; 13:354–59.
28). Kim YM, Oh CE, Kim SH, Lee J, Choi EH, Lee HJ. Nasal carriage of Staphylococcus aureus from healthy Children Attending Day Care Center. Korean J Pediatr Infect Dis. 2010; 17:9–15.
29). Lo WT, Lin WJ, Tseng MH, Lu JJ, Lee SY, Chu ML, et al. Nasal carriage of a single clone of community-acquired methicillin-resistant Staphylococcus aureus among kindergarten attendees in northern Taiwan. BMC Infect Dis. 2007; 7:51.

30). Lu PL, Chin LC, Peng CF, Chiang YH, Chen TP, Ma L, et al. Risk factors and molecular analysis of community methicillin-resistant Staphylococcus aureus carriage. J Clin Microbiol. 2005; 43:132–9.
31). Alfaro C, Mascher-Denen M, Fergie J, Purcell K. Prevalence of methicillin-resistant Staphylococcus aureus nasal carriage in patients admitted to Driscoll Children's Hospital. Pediatr Infect Dis J. 2006; 25:459–61.
32). Ko KS, Lee JY, Baek JY, Peck KR, Rhee JY, Kwon KT, et al. Characterization of Staphylococcus aureus nasal carriage from children attending an outpatient clinic in Seoul, Korea. Microb Drug Resist. 2008; 14:37–44.
33). Ko KS, Lee JY, Suh JY, Oh WS, Peck KR, Lee NY, et al. Distribution of major genotypes among methicillin-resistant Staphylococcus aureus clones in Asian countries. J Clin Microbiol. 2005; 43:421–6.
34). Lee J, Sung JY, Kim YM, Oh CE, Kim HB, Choi EH, et al. Molecular characterization of methicillin-resistant Staphylococcus aureus obtained from the anterior nares of healthy Korean children attending daycare centers. Int J Infect Dis. 2011; 15:558–63.
35). Kim TS, Kim MJ, Kim SH, Kee HY, Seo JJ, Kim ES, et al. Profile of Antimicrobial resistance of Staphylococcus aureus and molecular epidemiologic characterization of methicillin-resistant Staphylococcus aureus (MRSA) isolated from hands of people using multitude facilities. Infect Chemother. 2012; 44:289–98.
36). Vandenesch F, Naimi T, Enright MC, Lina G, Nimmo GR, Heffernan H, et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis. 2003; 9:978–84.
37). Song JH, Hsueh PR, Chung DR, Ko KS, Kang CI, Peck KR, et al. Spread of methicillin-resistant Staphylococcus aureus between the community and the hospitals in Asian countries: an ANSORP study. J Antimicrob Chemother. 2011; 66:1061–9.
38). Kim JS, Park JS, Song W, Kim HS, Cho HC, Lee KM, et al. Panton-Valentine Leukocidin Positive Staphylococcus aureus Isolated from Blood in Korea. Korean J Lab Med. 2007; 27:286–91.
39). McDougal LK, Steward CD, Killgore GE, Chaitram JM, McAllister SK, Tenover FC. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J Clin Microbiol. 2003; 41:5113–20.
Go to : 
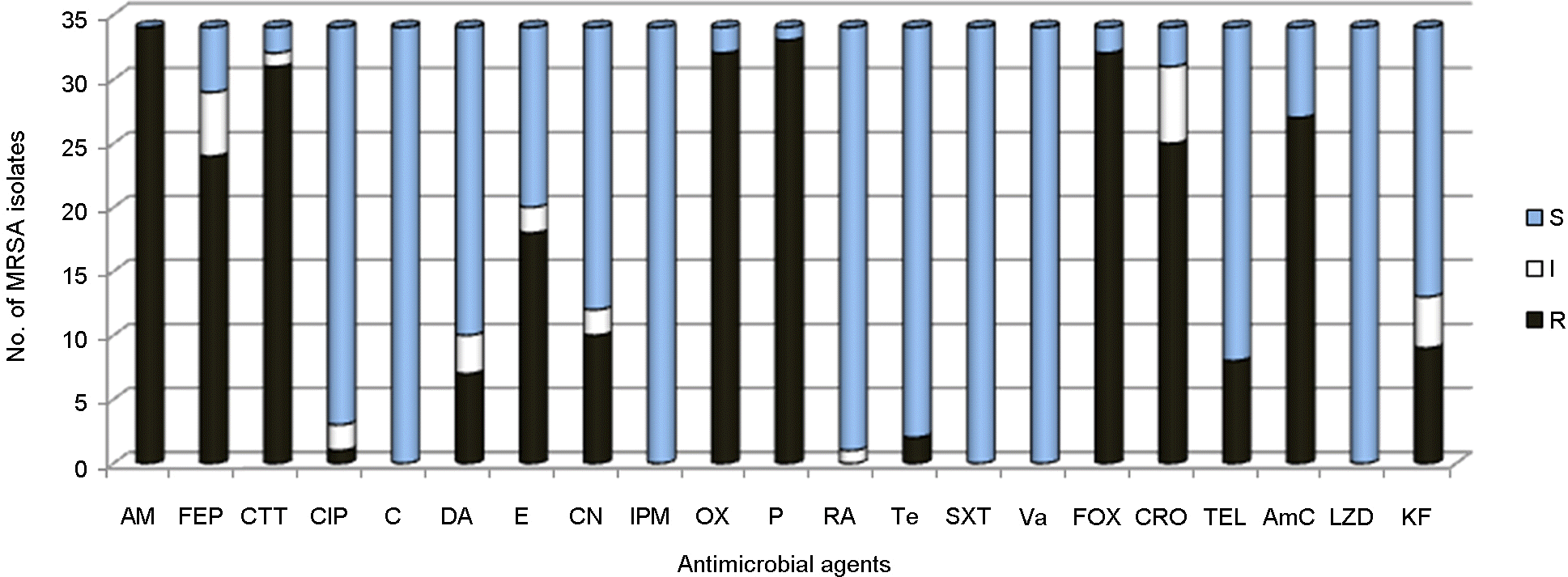 | Figure 1.Antimicrobial resistance patterns of community-associated methicillin-resistant S. aureus (CA-MRSA) isolates. S, susceptible; I, intermediate; R, resistant; AM ampicillin; FEP cefepime; CTT cefotetan; CIP ciprofloxacinem; C chloramphenicol; CC clindamycin; E erythromycin; CN amikacin; IPM imipenem; OX oxacillin; P penicillin; RA rifampin; TE tetracycline; SXT trimethoprim/sulfamethoxazole; VA vancomycin; FOX cefoxitin; CRO ceftriaxone; TEL telithromycin; AmC amoxicillin/clavulanic; LZD linenzolid; KF, cephalothin |
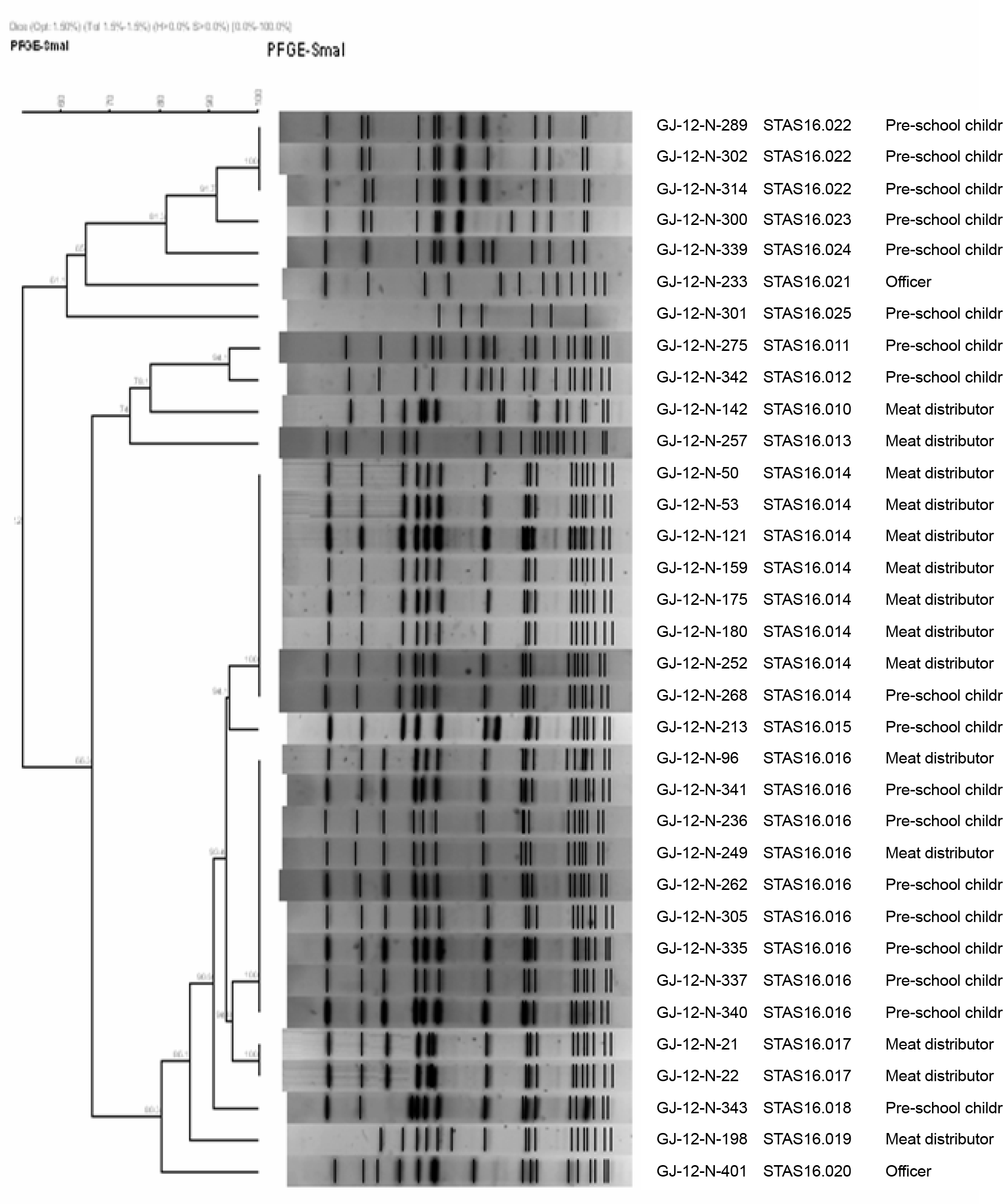 | Figure 2.Dendrogram of Sma I-digested pulsed-field gel electrophoresis (PFGE) profiles of community-associated methicillin-resistant S. aureus (CA-MRSA) isolates. |
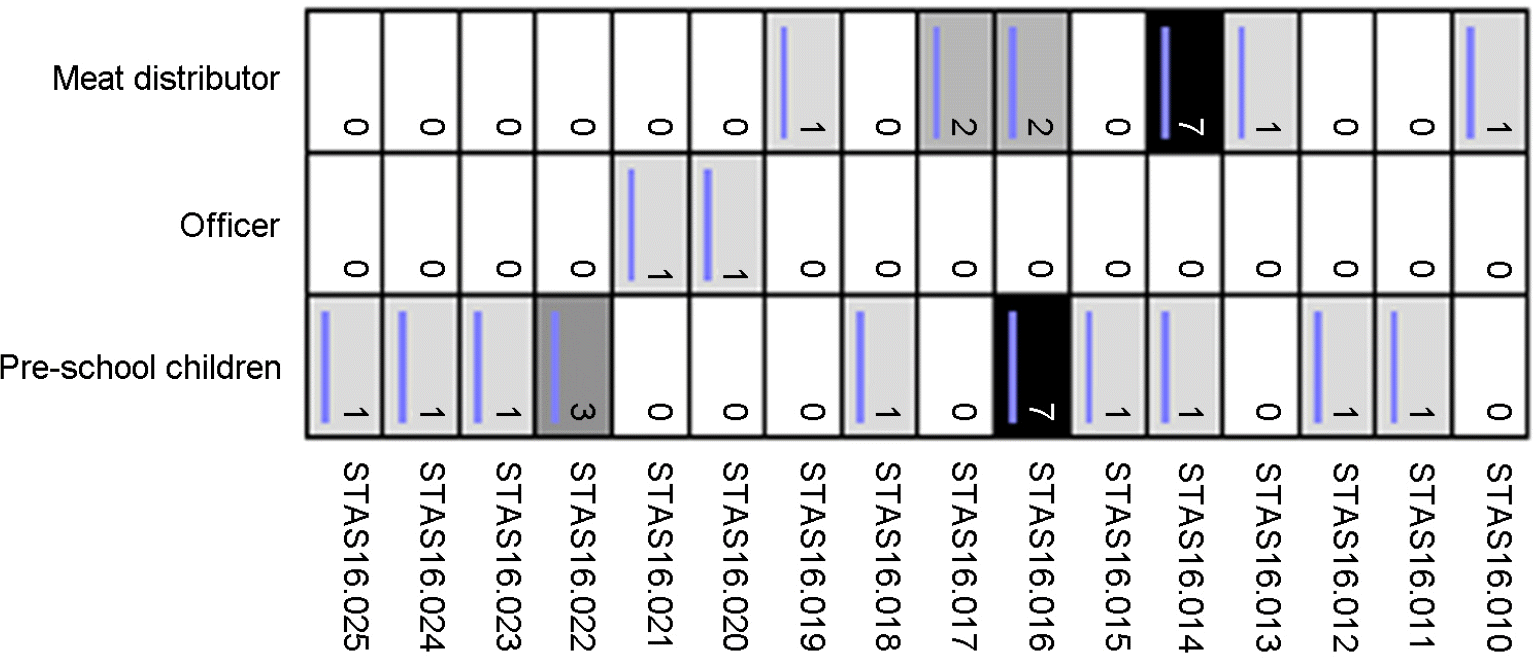 | Figure 3.Pulsed-field gel electrophoresis (PFGE) Sma I-pattern of community-associated methicillin-resistant S. aureus (CA-MRSA) isolates. |
Table 1.
Primers used in the multiplex polymerase chain reaction (PCR)
| Name | SCCmeca primer Sequence (5′ → 3′) | Target | Length | SCCmeca type |
|---|---|---|---|---|
| B | ATTGCCTTGATAATAGCCYTCT | ccrAs-B | 937 bp | II, IV |
| a3 | TAAAGGCATCAATGCACAAACACT | |||
| ccrCF | CGTCTATTACAAGATGTTAAGGATAAT | |||
| ccrCR | CCTTTATAGACTGGATTATTCAAAATAT | ccrC | 518 bp | III, V |
| 1272F1 | GCCACTCATAACATATGGAA | |||
| 1272R1 | CATCCGAGTGAAACCCAAA | IS1272 | 415 bp | I, IV |
| 5RmecA | TATACCAAACCCGACAACTAC | |||
| 5R431 | CGGCTACAGTGATAACATCC | mecA/IS431 | 359 bp | V |
| Name | PVLb toxin primer Sequence (5′ → 3′) | Length | ||
| luk-PV-1 | ATCATTAGGTAAAATGTCTGGACATGATCCA | |||
| luk-PV-2 | GCATCAASTGTATTGGATAGCAAAAGC | 433 bp |
Table 2.
Prevalence of community-associated methicillin-resistant S. aureus (CA-MRSA) isolates from meat distributors, pre-school children and officers
Table 3.
Antimicrobial resistance patterns of community-associated methicillin-resistant S. aureus (CA-MRSA) isolates
AM, Ampicillin; FEP, Cefepime; CTT, Cefotetan; CIP, Ciprofloxacinem; C, Chloramphenicol; CC, Clindamycin; E, Erythromycin; CN, Amikacin; IPM, Imipenem; OX, Oxacillin; P, Penicillin; RA, Rifampin; TE, Tetracycline; SXT, Trimethoprim/Sulfamethoxazole; VA, Vancomycin; FOX, Cefoxitin; CRO, Ceftriaxone; TEL, Telithromycin; AmC, Amoxicillin/Clavulanic; LZD, Linenzolid; KF, Cephalothin
Table 4.
Characteristics of community-associated methicillin-resistant S. aureus (CA-MRSA) isolates from nasal swabs
| Strain | Characteristics |
MIC (μg/ml) |
mecAa | PVLa | SCCmecb | PFGE |
|---|---|---|---|---|---|---|
| OX | ||||||
| GJ-12-N-21 | Meat distributor | 64 | + | − | IV | STAS16.017 |
| GJ-12-N-22 | Meat distributor | 64 | + | − | IV | STAS16.017 |
| GJ-12-N-50 | Meat distributor | 48 | + | − | IV | STAS16.014 |
| GJ-12-N-53 | Meat distributor | 64 | + | − | IV | STAS16.014 |
| GJ-12-N-96 | Meat distributor | 64 | + | − | IV | STAS16.016 |
| GJ-12-N-121 | Meat distributor | 64 | + | − | IV | STAS16.014 |
| GJ-12-N-142 | Meat distributor | 0.75 | + | − | − | STAS16.010 |
| GJ-12-N-159 | Meat distributor | 64 | + | − | IV | STAS16.014 |
| GJ-12-N-175 | Meat distributor | 64 | + | − | IV | STAS16.014 |
| GJ-12-N-180 | Meat distributor | 64 | + | − | IV | STAS16.014 |
| GJ-12-N-198 | Meat distributor | 48 | + | − | IV | STAS16.019 |
| GJ-12-N-213 | Pre-school children | 64 | + | − | IV | STAS16.015 |
| GJ-12-N-233 | Officer | 0.25 | + | − | − | STAS16.021 |
| GJ-12-N-236 | Pre-school children | 64 | + | − | IV | STAS16.016 |
| GJ-12-N-249 | Meat distributor | 48 | + | − | IV | STAS16.016 |
| GJ-12-N-252 | Meat distributor | 128 | + | − | IV | STAS16.014 |
| GJ-12-N-257 | Meat distributor | 64 | + | − | III | STAS16.013 |
| GJ-12-N-262 | Pre-school children | 64 | + | − | IV | STAS16.016 |
| GJ-12-N-268 | Pre-school children | 64 | + | − | IV | STAS16.014 |
| GJ-12-N-275 | Pre-school children | 128 | + | − | IV | STAS16.011 |
| GJ-12-N-289 | Pre-school children | 48 | + | − | II | STAS16.022 |
| GJ-12-N-300 | Pre-school children | 128 | + | − | II | STAS16.023 |
| GJ-12-N-301 | Pre-school children | 64 | + | − | II | STAS16.025 |
| GJ-12-N-302 | Pre-school children | 96 | + | − | II | STAS16.022 |
| GJ-12-N-305 | Pre-school children | 64 | + | − | IV | STAS16.016 |
| GJ-12-N-314 | Pre-school children | 64 | + | − | II | STAS16.022 |
| GJ-12-N-335 | Pre-school children | 64 | + | − | IV | STAS16.016 |
| GJ-12-N-337 | Pre-school children | 64 | + | − | IV | STAS16.016 |
| GJ-12-N-339 | Pre-school children | 96 | + | − | IV | STAS16.024 |
| GJ-12-N-340 | Pre-school children | 96 | + | − | IV | STAS16.016 |
| GJ-12-N-341 | Pre-school children | 96 | + | − | IV | STAS16.016 |
| GJ-12-N-342 | Pre-school children | 32 | + | − | II | STAS16.012 |
| GJ-12-N-343 | Pre-school children | 64 | + | − | IV | STAS16.018 |
| GJ-12-N-401 | Officer | 32 | + | − | IV | STAS16.020 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download