Abstract
Nucleoside diphosphate kinase (Ndk) is ubiquitous and highly conserved multifunctional key enzyme in nucleotide metabolism. It generates nucleoside triphosphates (NTPs) by transfer of gamma-phosphates from nucleoside triphosphates such as ATP or GTP to nucleoside diphosphate. The formation of an autophosphorylated enzyme intermediate is involved in that mechanism. The phosphate is usually supplied by ATP and Ndk activity in different subcellular compartments. Ndk may regulate the crucial balance between ATP and GTP or other nucleoside triphosphates. Ndk is playing an important role in bacterial pathogenesis and emerging evidences recognize multiple roles of Ndk in host-microbe interaction. Here, I review some examples of the role of Ndk in intra- and extracellular microorganism.
REFERENCES
1). Clark VL, Bavoil PM. Bacterial pathogenesis, part A: identification and regulation of virulence factors. Methods in Enzymology. San Diego: Academic Press;vol. 235. 1994.
2). Chakrabarty AM. Nucleoside diphosphate kinase: role in bacterial growth, virulence, cell signalling and polysaccharide synthesis. Mol Microbiol. 1998; 28:875–82.

3). Dar HH, Prasad D, Varshney GC, Chakraborti PK. Secretory nucleoside diphosphate kinases from both intra- and extracellular pathogenic bacteria are functionally indistinguishable. Microbiology. 2011; 157:3024–35.

4). Punj V, Zaborina O, Dhiman N, Falzari K, Bagdasarian M, Chakrabarty AM. Phagocytic cell killing mediated by secreted cytotoxic factors of Vibrio cholerae. Infect Immun. 2000; 68:4930–7.
5). Melnikov A, Zaborina O, Dhiman N, Prabhakar BS, Chakrabarty AM, Hendrickson W. Clinical and environmental isolates of Burkholderia cepacia exhibit differential cytotoxicity towards macrophages and mast cells. Mol Microbiol. 2000; 36:1481–93.
6). Yilmaz O, Yao L, Maeda K, Rose TM, Lewis EL, Duman M, et al. ATP scavenging by the intracellular pathogen Porphyromonas gingivalis inhibits P2X7-mediated host-cell apoptosis. Cell Microbiol. 2008; 10:863–75.
7). Kolli BK, Kostal J, Zaborina O, Chakrabarty AM, Chang KP. Leishmania-released nucleoside diphosphate kinase prevents ATP-mediated cytolysis of macrophages. Mol Biochem Parasitol. 2008; 158:163–75.
8). Zaborina O, Dhiman N, Ling Chen M, Kostal J, Holder IA, Chakrabarty AM. Secreted products of a nonmucoid Pseudomonas aeruginosa strain induce two modes of macrophage killing: external-ATP-dependent, P2Z-receptor-mediated necrosis and ATP-independent, caspase-mediated apoptosis. Microbiology. 2000; 146:2521–30.
9). Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006; 112:358–404.

10). Ali SR, Timmer AM, Bilgrami S, Park EJ, Eckmann L, Nizet V, et al. Anthrax toxin induces macrophage death by p38 MAPK inhibition but leads to inflammasome activation via ATP leakage. Immunity. 2011; 35:34–44.

11). Pedra JH, Cassel SL, Sutterwala FS. Sensing pathogens and danger signals by the inflammasome. Curr Opin Immunol. 2009; 21:10–6.

12). Ramachandra L, Qu Y, Wang Y, Lewis CJ, Cobb BA, Takatsu K, et al. Mycobacterium tuberculosis synergizes with ATP to induce release of microvesicles and exosomes containing major histocompatibility complex class II molecules capable of antigen presentation. Infect Immun. 2010; 78:5116–25.
13). Spooner R, Yilmaz O. The role of reactive-oxygen-species in microbial persistence and inflammation. Int J Mol Sci. 2011; 12:334–52.

14). Fontanils U, Seil M, Pochet S, El Ouaaliti M, Garcia-Marcos M, Dehaye JP, et al. Stimulation by P2X(7) receptors of calcium-dependent production of reactive oxygen species (ROS) in rat submandibular glands. Biochim Biophys Acta. 2010; 1800:1183–91.

15). Yilmaz O, Verbeke P, Lamont RJ, Ojcius DM. Intercellular spreading of Porphyromonas gingivalis infection in primary gingival epithelial cells. Infect Immun. 2006; 74:703–10.
16). Meena LS, Chopra P, Bedwal RS, Singh Y. Nucleoside diphosphate kinase-like activity in adenylate kinase of Mycobacterium tuberculosis. Biotechnol Appl Biochem. 2003; 38:169–74.
17). Sureka K, Sanyal S, Basu J, Kundu M. Polyphosphate kinase 2: a modulator of nucleoside diphosphate kinase activity in mycobacteria. Mol Microbiol. 2009; 74:1187–97.

18). Huynh KK, Plumb JD, Downey GP, Valvano MA, Grinstein S. Inactivation of macrophage Rab7 by Burkholderia cenocepacia. J Innate Immun. 2010; 2:522–33.
19). Keith KE, Hynes DW, Sholdice JE, Valvano MA. Delayed association of the NADPH oxidase complex with macrophage vacuoles containing the opportunistic pathogen Burkholderia cenocepacia. Microbiology. 2009; 155:1004–15.
21). Meena LS, Rajni . Survival mechanisms of pathogenic Mycobacterium tuberculosis H37Rv. FEBS J. 2010; 277:2416–27.
22). Sun J, Wang X, Lau A, Liao TY, Bucci C, Hmama Z. Mycobacterial nucleoside diphosphate kinase blocks phagosome maturation in murine RAW 264.7 macrophages. PLoS One. 2010; 5:e8769.

23). Saini AK, Maithal K, Chand P, Chowdhury S, Vohra R, Goyal A, et al. Nuclear localization and in situ DNA damage by Mycobacterium tuberculosis nucleoside-diphosphate kinase. J Biol Chem. 2004; 279:50142–9.
24). Epting CL, Coates BM, Engman DM. Molecular mechanisms of host cell invasion by Trypanosoma cruzi. Exp Parasitol. 2010; 126:283–91.
25). Mantuano-Barradas M, Henriques-Pons A, Araújo-Jorge TC, Di Virgilio F, Coutinho-Silva R, Persechini PM. Extracellular ATP induces cell death in CD4+/CD8+ double-positive thymocytes in mice infected with Trypanosoma cruzi. Microbes Infect. 2003; 5:1363–71.
26). Wolf M, Müller T, Dandekar T, Pollack JD. Phylogeny of Firmicutes with special reference to Mycoplasma (Mollicutes) as inferred from phosphoglycerate kinase amino acid sequence data. Int J Syst Evol Microbiol. 2004; 54:871–5.

27). Hunger-Glaser I, Hemphill A, Shalaby T, Hänni M, Seebeck T. Nucleoside diphosphate kinase of Trypanosoma brucei. Gene. 2000; 257:251–7.
28). Levit MN, Abramczyk BM, Stock JB, Postel EH. Interactions between Escherichia coli nucleoside-diphosphate kinase and DNA. J Biol Chem. 2002; 277:5163–7.
29). Corrêa G, Marques da Silva C, de Abreu Moreira-Souza AC, Vommaro RC, Coutinho-Silva R. Activation of the P2X(7) receptor triggers the elimination of Toxoplasma gondii tachyzoites from infected macrophages. Microbes Infect. 2010; 12:497–504.
30). Kowluru A, Veluthakal R, Kaetzel DM. Regulatory roles for nm23/nucleoside diphosphate kinase-like enzymes in insulin secretion from the pancreatic islet beta cell. J Bioenerg Biomembr. 2006; 38:227–32.

31). Vu ND, Wagner PD. Stimulation of secretion in permeabilized PC12 cells by adenosine 5′-[gamma-thio]triphosphate: possible involvement of nucleoside diphosphate kinase. Biochem J. 1993; 296:169–74.
32). Zaborina O, Li X, Cheng G, Kapatral V, Chakrabarty AM. Secretion of ATP-utilizing enzymes, nucleoside diphosphate kinase and ATPase, by Mycobacterium bovis BCG: sequestration of ATP from macrophage P2Z receptors? Mol Microbiol. 1999; 31:1333–43.
33). Kamath S, Chen ML, Chakrabarty AM. Secretion of nucleoside diphosphate kinase by mucoid Pseudomonas aeruginosa 8821: involvement of a carboxy-terminal motif in secretion. J Bacteriol. 2000; 182:3826–31.
34). Nickel W. The mystery of nonclassical protein secretion. A current view on cargo proteins and potential export routes. Eur J Biochem. 2003; 270:2109–19.
35). Pollak CN, Delpino MV, Fossati CA, Baldi PC. Outer membrane vesicles from Brucella abortus promote bacterial internalization by human monocytes and modulate their innate immune response. PLoS One. 2012; 7:e50214.
36). Manning AJ, Kuehn MJ. Contribution of bacterial outer membrane vesicles to innate bacterial defense. BMC Microbiol. 2011; 11:258.

37). Bomberger JM, Maceachran DP, Coutermarsh BA, Ye S, O'Toole GA, Stanton BA. Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog. 2009; 5:e1000382.
38). Xiong H, Li S, Yang Z, Burgess RR, Dynan WS. E. coli expression of a soluble, active single-chain antibody variable fragment containing a nuclear localization signal. Protein Expr Purif. 2009; 66:172–80.
39). Mukhopadhyay A, Ni L, Yang CS, Weiner H. Bacterial signal peptide recognizes HeLa cell mitochondrial import receptors and functions as a mitochondrial leader sequence. Cell Mol Life Sci. 2005; 62:1890–9.

40). Berleman J, Auer M. The role of bacterial outer membrane vesicles for intra- and interspecies delivery. Environ Microbiol. 2013; 15:347–54.

41). Kuehn MJ, Kesty NC. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 2005; 19:2645–55.

Figure 1.
Sequence alignment of nucleoside diphosphate kinase in different microorganisms. Sequence alignment was performed using Clustal/W.
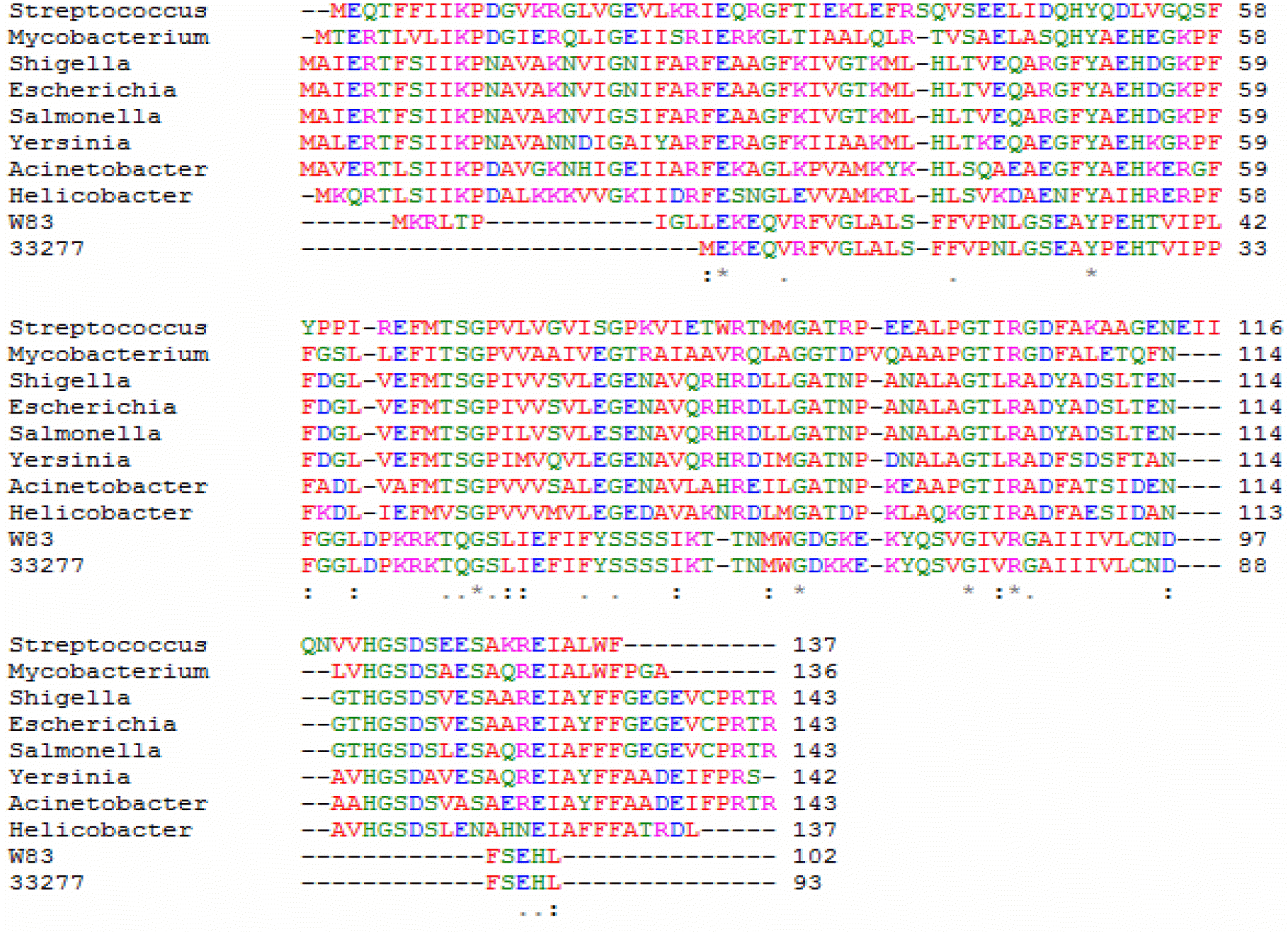
Figure 2.
Schematic diagram of potential functions of Ndk and eATP-P2X receptor signaling in possible exploitation by intracellular pathogens for release of Ndk outside of host cells. Translocation of Ndk through cell membrane channels to extracellular environment (A). Association of Ndk with host ER-derived vesicle and consequent export (B). Simultaneous integration of Ndk within microbe secretory vesicles that fuse with host cell membrane and release contents (C).
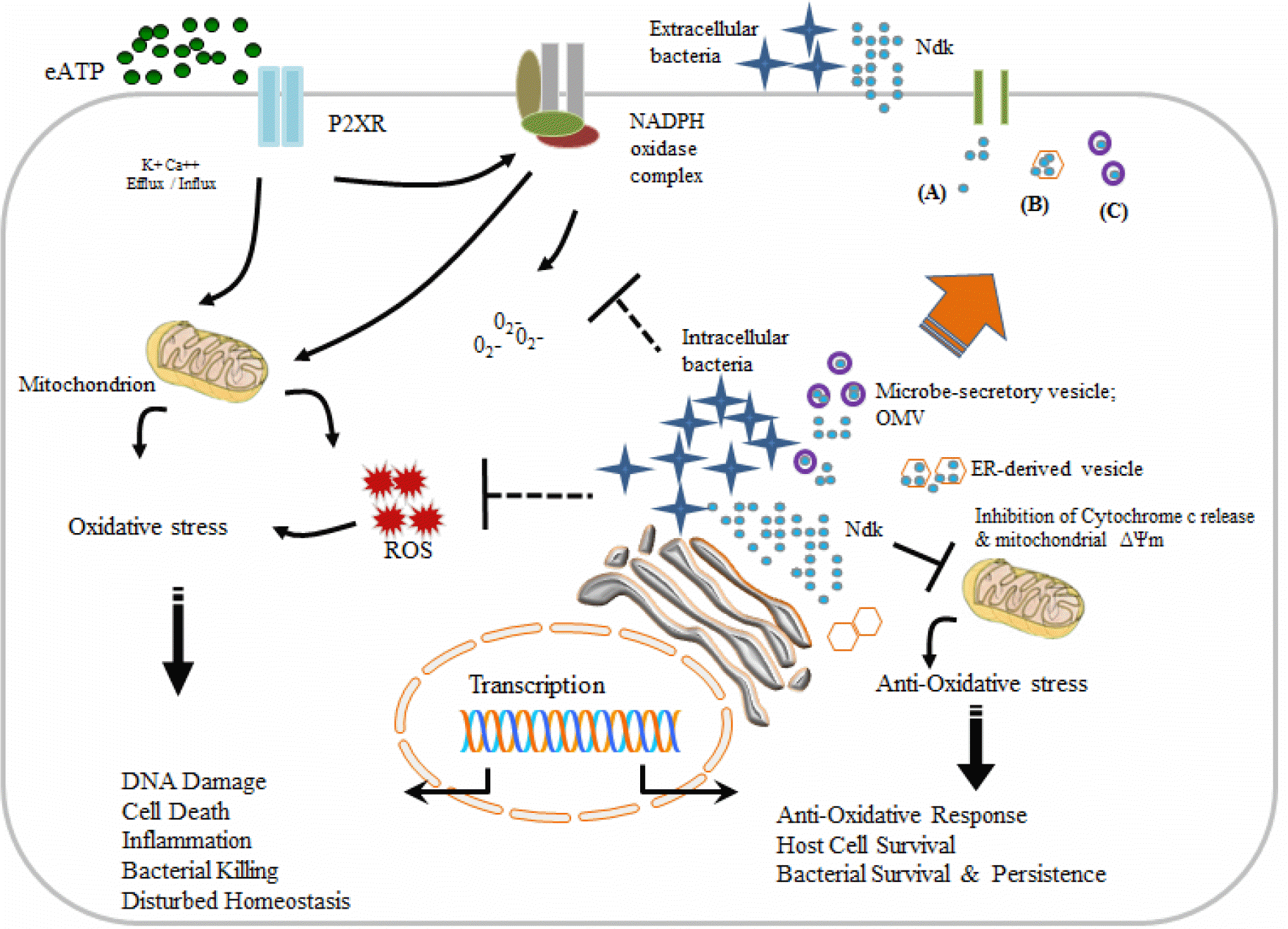
Table 1.
Utilization of Ndk in host-microbe interaction




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download