Abstract
We have previously observed that a sequence in coat protein (CP) ORF of Turnip yellow mosaic virus (TYMV) is required for efficient replication of the virus. The sequence was predicted to take a stem-loop structure, thus termed SL2. While examining various SL2 mutants, we observed that all the modifications resulting in extension of translation beyond the CP ORF significantly suppressed subgenomic RNA accumulation. The genomic RNA level, in contrast, was not affected. Introduction of an in-frame stop codon in the CP ORF of these constructs restored the level of subgenomic RNA. Overall, the results suggest that the read-through makes the subgenomic RNA unstable.
Go to : 
REFERENCES
1). Dreher TW. Turnip yellow mosaic virus: transfer RNA mimicry, chloroplasts and a C-rich genome. Mol Plant Pathol. 2004; 5:367–75.

2). Matsuda D, Dreher TW. The tRNA-like structure of Turnip yellow mosaic virus RNA is a 3′-translational enhancer. Virology. 2004; 321:36–46.

3). Matsuda D, Bauer L, Tinnesand K, Dreher TW. Expression of the two nested overlapping reading frames of Turnip yellow mosaic virus RNA is enhanced by a 5′ cap and by 5′ and 3′ viral sequences. J Virol. 2004; 78:9325–35.

4). Matthews REF. Tymovirus group. In Plant Virology. 3rd ed.San Diego: Academic Press;;1991. p. 231–9.
5). Prod'homme D, Jakubiec A, Tournier V, Drugeon G, Jupin I. Targeting of the turnip yellow mosaic virus 66K replication protein to the chloroplast envelope is mediated by the 140K protein. J Virol. 2003; 77:9124–35.
6). Jakubiec A, Notaise J, Tournier V, Héricourt F, Block MA, Drugeon G, et al. Assembly of turnip yellow mosaic virus replication complexes: Interaction between the proteinase and polymerase domains of the replication proteins. J Virol. 2004; 78:7945–57.
7). Hellendoorn K, Michiels PJ, Buitenhuis R, Pleij CW. Protonatable hairpins are conserved in the 5′-untranslated region of tymovirus RNAs. Nucleic Acids Res. 1996; 24:4910–7.

8). Hellendoorn K, Verlaan PW, Pleij CW. A functional role for the conserved protonatable hairpins in the 5′ untranslated region of turnip yellow mosaic virus RNA. J Virol. 1997; 71:8774–9.

9). Shin HI, Tzanetakis IE, Dreher TW, Cho TJ. The 5′-UTR of Turnip yellow mosaic virus does not include a critical encapsidation signal. Virology. 2009; 387:427–35.

10). Deiman BA, Koenen AK, Verlaan PW, Pleij CW. Minimal template requirements for initiation of minus-strand synthesis in vitro by the RNA-dependent RNA polymerase of turnip yellow mosaic virus. J Virol. 1998; 72:3965–72.
11). Deiman BA, Verlaan PW, Pleij CW. In vitro transcription by the turnip yellow mosaic virus RNA polymerase: a comparison with the alfalfa mosaic virus and brome mosaic virus replicases. J Virol. 2000; 74:264–71.
12). Singh RN, Dreher TW. Specific site selection in RNA resulting from a combination of nonspecific secondary structure and -CCR- boxes: initiation of minus strand synthesis by turnip yellow mosaic virus RNA-dependent RNA polymerase. RNA. 1998; 4:1083–95.

13). Shin HI, Kim IC, Cho TJ. Replication and encapsidation of recombinant Turnip yellow mosaic virus RNA. BMB Rep. 2008; 41:739–44.
14). Shin HI, Cho TJ. A sequence in coat protein open reading frame is required for Turnip yellow mosaic virus replication. J Bacteriol Virol. 2011; 41:109–16.

15). Shin HI, Cho TJ. Characterization of a replication element in the coat protein ORF of Turnip yellow mosaic virus. J Bacteriol Virol. 2012; 42:49–55.

16). Cho TJ, Dreher TW. Encapsidation of genomic but not subgenomic turnip yellow mosaic virus RNA by coat protein provided in trans. Virology. 2006; 356:126–35.

17). Miller WA, White KA. Long-distance RNA-RNA interactions in plant virus gene expression and replication. Annu Rev Phytopathol. 2006; 44:447–67.

18). Pogany J, White KA, Nagy PD. Specific binding of tombusvirus replication protein p33 to an internal replication element in the viral RNA is essential for replication. J Virol. 2005; 79:4859–69.

19). Dreher TW. Functions of the 3′-untranslated regions of positive strand RNA viral genomes. Annu Rev Phytopathol. 1999; 37:151–74.

20). Dreher TW, Uhlenbeck OC, Browning KS. Quantitative assessment of EF-1alpha. GTP binding to aminoacyl-tRNAs, aminoacyl-viral RNA, and tRNA shows close correspondence to the RNA binding properties of EF-Tu. J Biol Chem. 1999; 274:666–72.
Go to : 
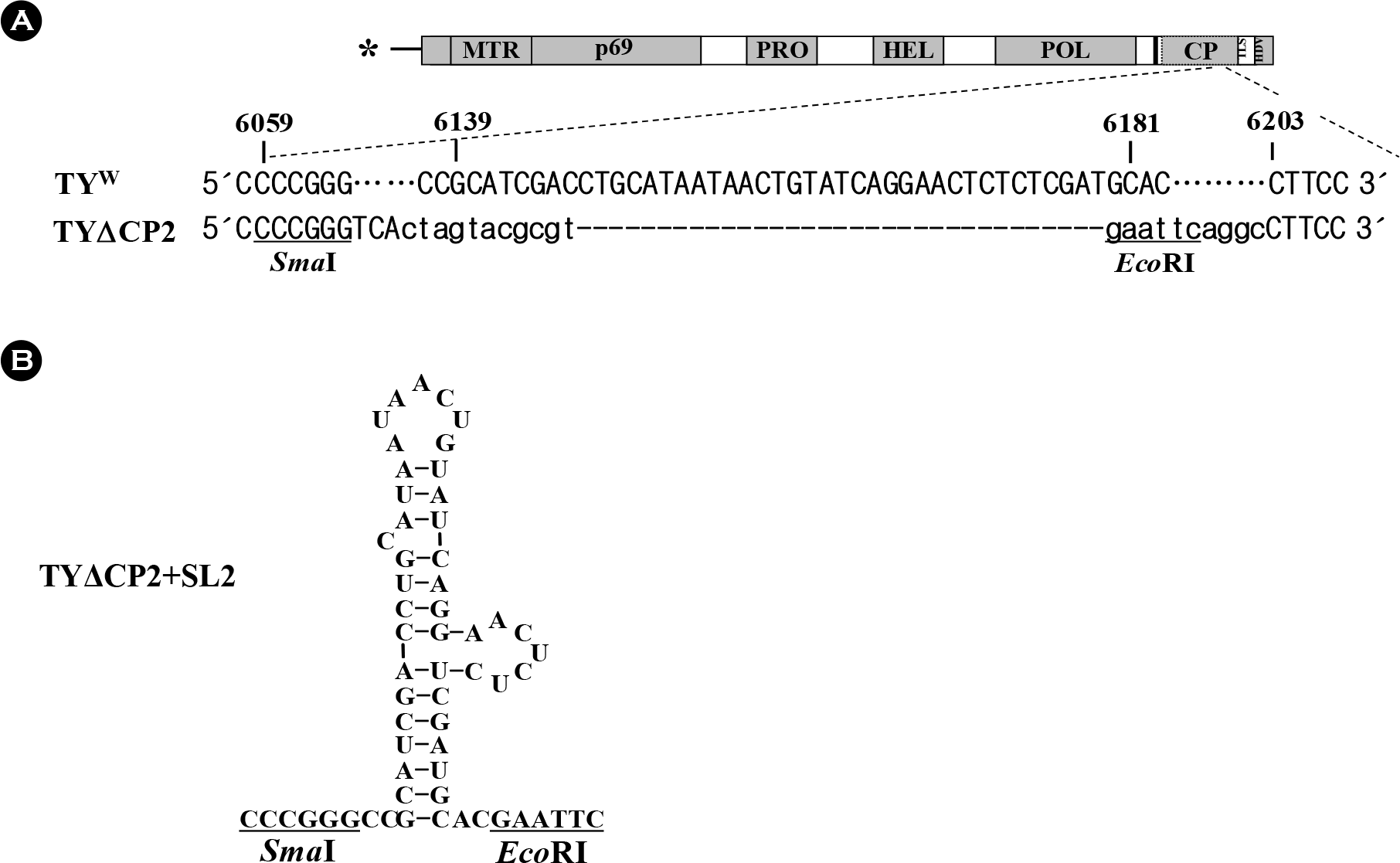 | Figure 1.TYΔCP2 and SL2 constructs. (A) TYMV genome and a deletion construct TYΔCP2. TYMV genome is schematically represented with the predicted domains: MTR, methyltransferase; PRO, protease; HEL, helicase; POL, polymerase; CP, coat protein. The sequence between nt-6139 and nt-6181 in wild-type TYMV (TYW) is predicted to take a stem-loop structure, termed SL2. In TYΔCP2, the nucleotides between nt-6067 and nt-6202 were deleted and several cloning sites were added (SmaI and EcoRI recognition sites are underlined). Nonviral sequences are indicated by small letters. (B) TYΔCP2+SL2 construct. In this construct, the sequence between nt-6137 and nt-6183 was added back into the TYΔCP2. Predicted secondary structure of SL2 is represented. |
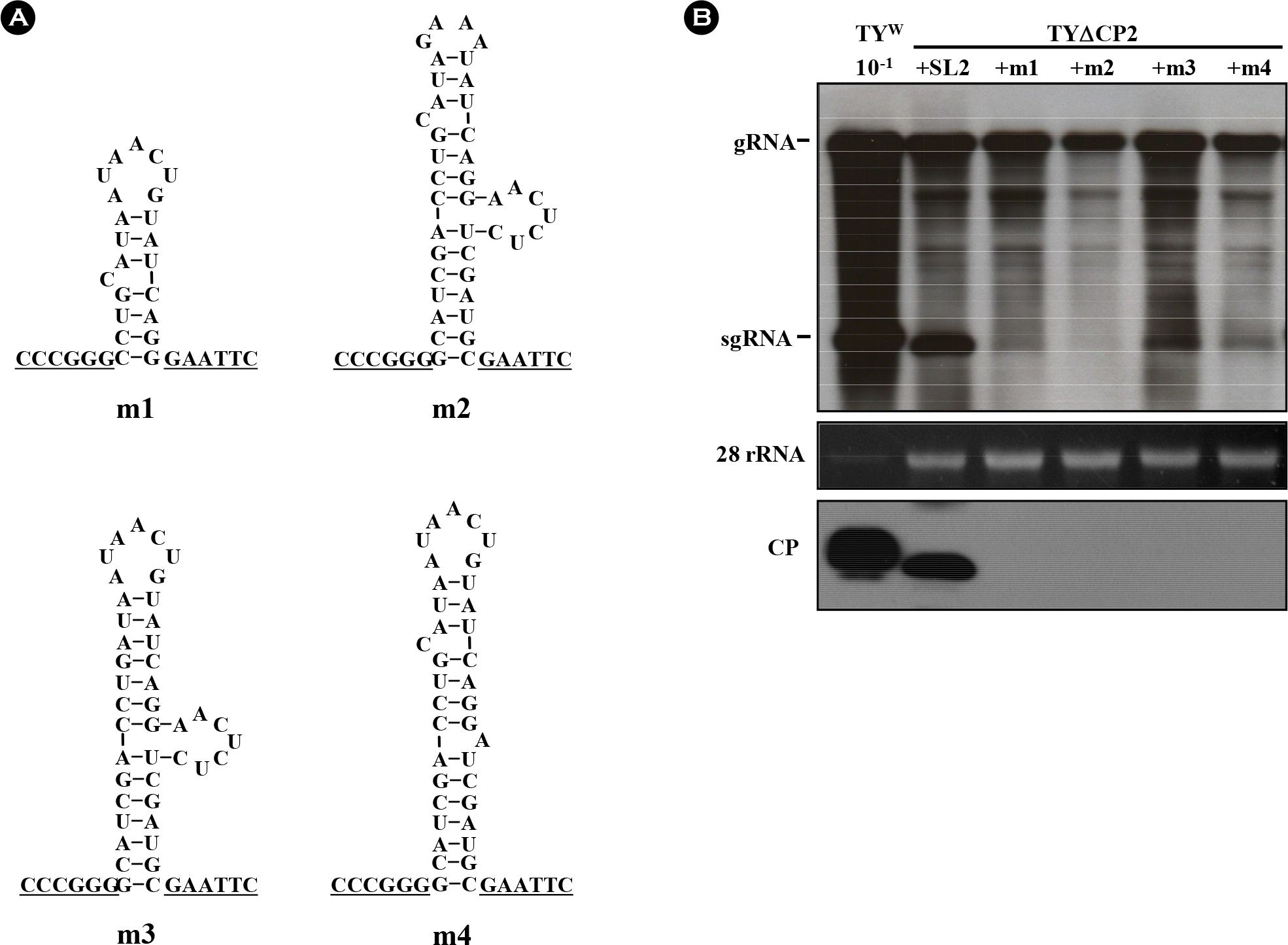 | Figure 2.Influence of SL2 modifications on TYMV replication. (A) Variant SL2 constructs. TYΔCP2+m1 lacks the bottom half of the stem. In TYΔCP2+m2, the terminal loop was replaced with a GAAA tetraloop. In TYΔCP2+m3, the non-paired C in the upper stem was deleted. In TYΔCP2+m4, the side bulge was replaced with a single A. (B) Northern and Western analysis of the SL2 mutants. Seven days after agroinfiltration of N. benthamiana leaf with various TYMV constructs, total RNA was extracted from the leaf. 1 μg or 0.1 μg (10−1) of total RNA was size-fractionated in the 1% agarose gel and examined by Northern blot analysis, using the DIG-labeled probe representing the CP ORF. The blots were developed by chemiluminescent immunodetection of DIG. The panel below the Northern blot represents a gel stained with ethidium bromide. In Western blot analysis of coat protein expression (bottom panel), 1 μl of 1:10 diluted (10−1) or undiluted leaf extract was loaded and electrophoresed in 12.5% SDS-polyacrylamide gel. The proteins were transferred to a nitrocellulose membrane. Coat protein was detected using anti-TYMV coat protein rabbit antibody and anti-rabbit HRP conjugate. The membrane was developed by a Luminata™ Forte (Millipore) using luminol as the substrate. |
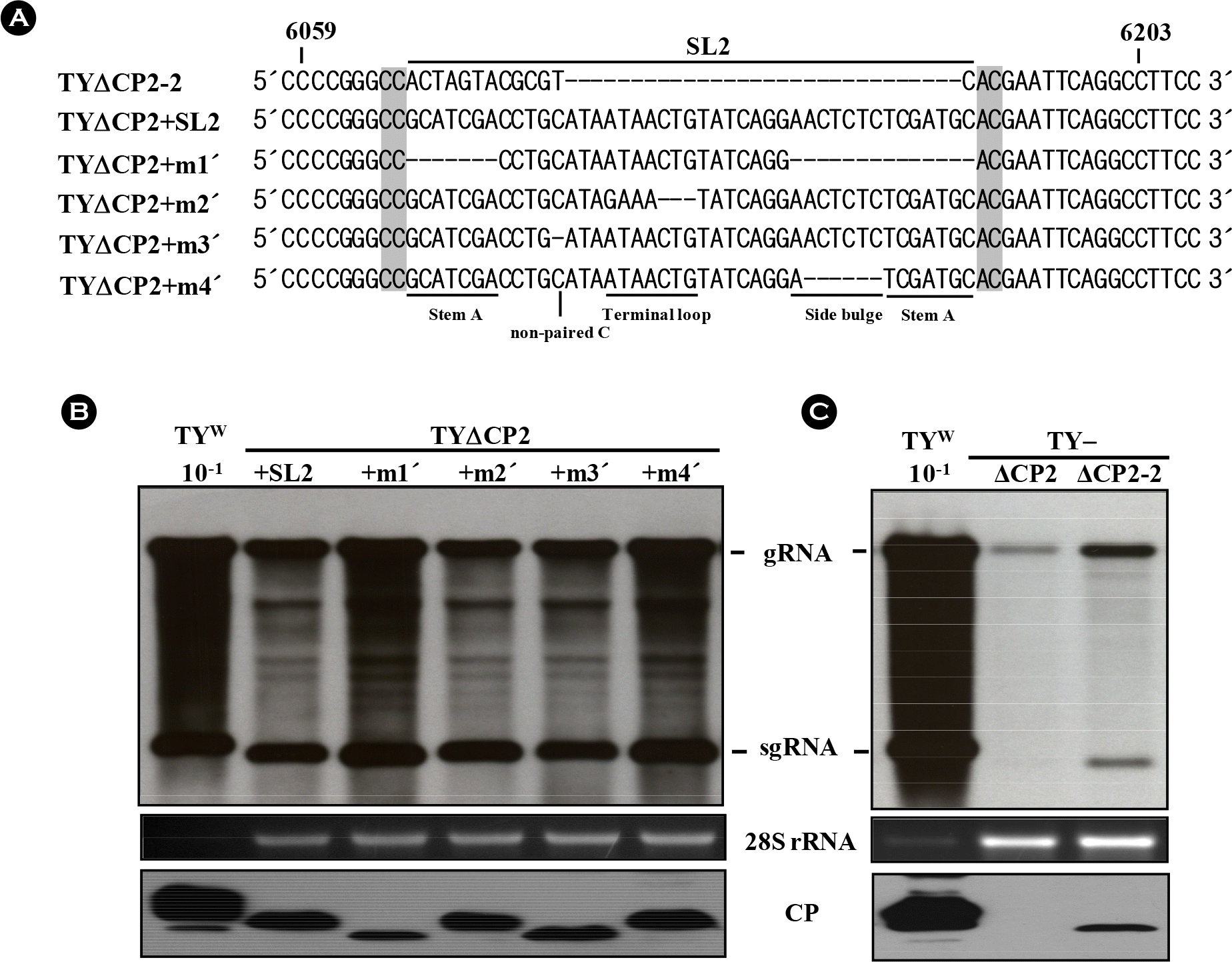 | Figure 3.Effect of the sequence adjacent to the SL2. (A) Alignment of the constructs derived from TYΔCP2. As in TYΔCP2+SL2, TYΔCP2+m1′∼TYΔCP2+m4′ constructs have CC and AC nucleotides upstream and downstream of the SL2 sequence. In contrast, the CC and AC nucleotides are missing in TYΔCP2+m1∼TYΔCP2+m4 constructs (see Fig. 2A). In TYΔCP2-2, the CC and AC nucleotides were added into the TYΔCP2. (B) Replication of the TYΔCP2+m1′∼TYΔCP2+m4′ constructs containing the CC/AC. (C) Replication of the TYΔCP2-2 containing CC/AC. Northern and western analyses were done as described in Fig. 2. |
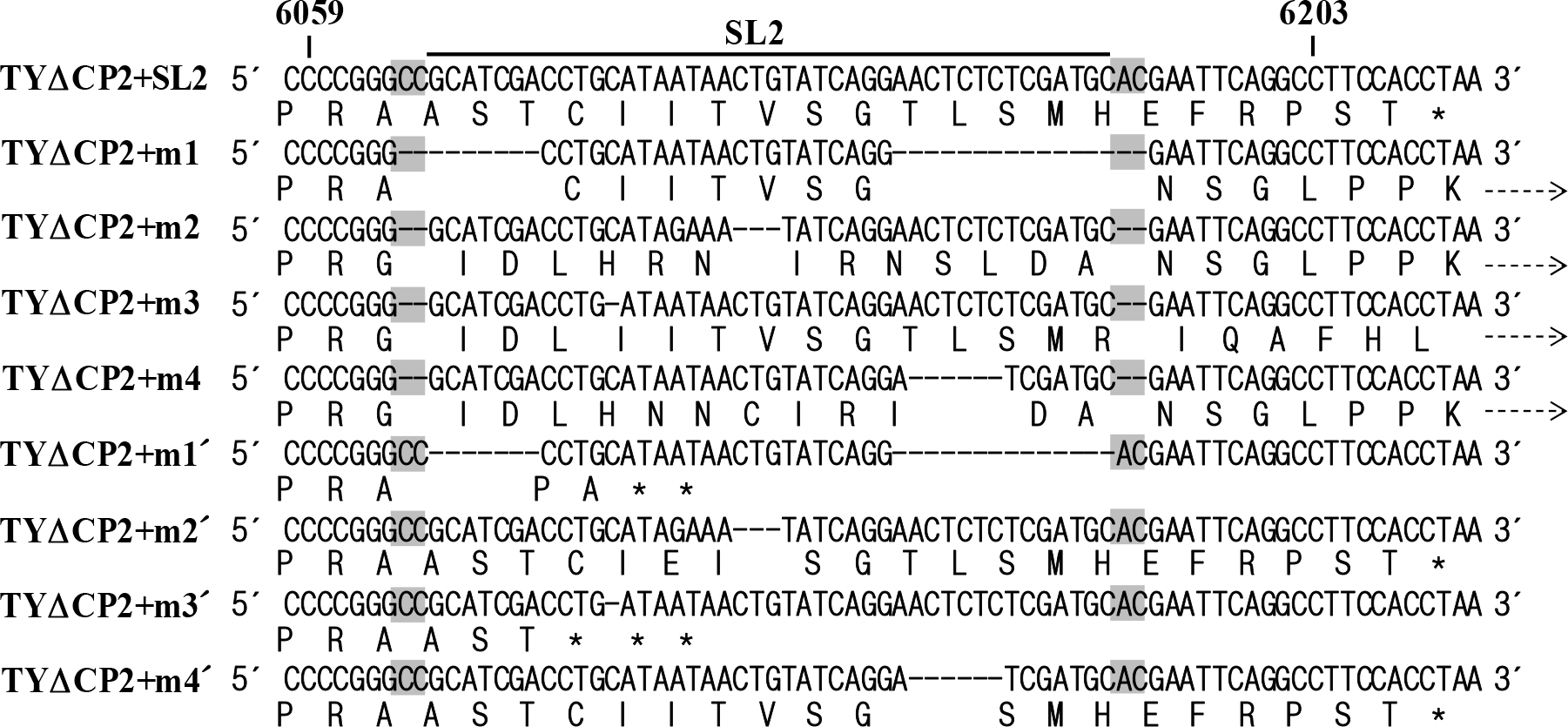 | Figure 4.Alignment of the predicted translation products from TYΔCP2-derived constructs. Nucleotide and predicted amino acid sequences of the TYΔCP2+SL2, TYΔCP2+m1∼TYΔCP2+m4, and TYΔCP2+m1′∼TYΔCP2+m4′ constructs are shown. Stop codons are indicated by asterisks. In TYΔCP2+m1∼TYΔCP2+m4 constructs, translation would be extended beyond the CP ORF up to the 3′-end. Broken arrows indicate read-through. 35 amino acids could be additionally added to the C-terminus of CP, compared to the TYΔCP2+SL2. |
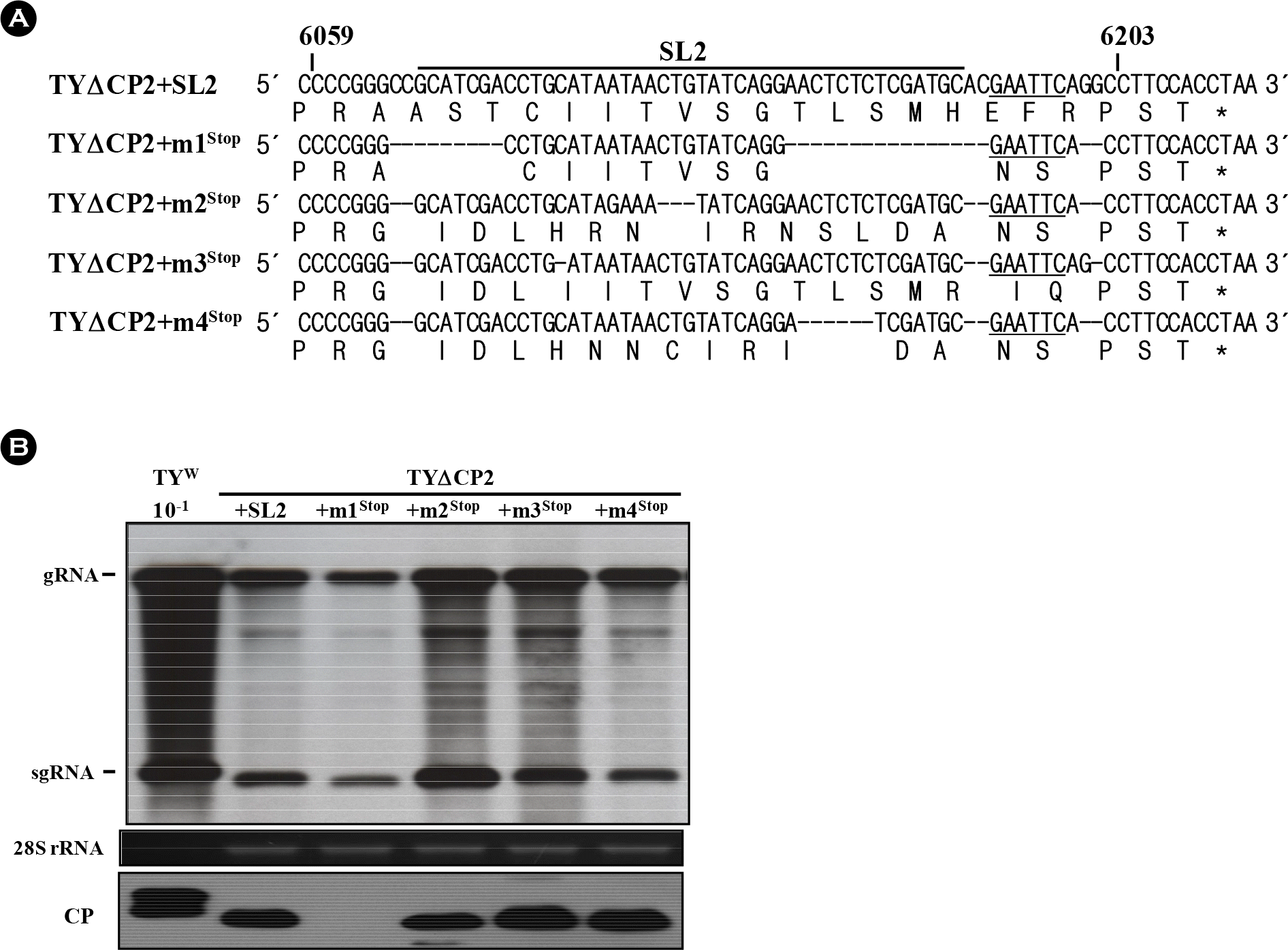 | Figure 5.Effect of in-frame stop codons on sgRNA accumulation. (A) Alignment of the TYΔCP2+m1∼TYΔCP2+m4 constructs having in-frame stop codons. In TYΔCP2+m1stop∼TYΔCP2+m4stop constructs, one or two nucleotides after the EcoRI site (underlined) were removed to have an in-frame stop codon as in TYΔCP2+SL2. (B) Replication of the TYΔCP2+m1stop∼TYΔCP2+m4stop constructs. Northern and western analyses were done as described in Fig. 2. |
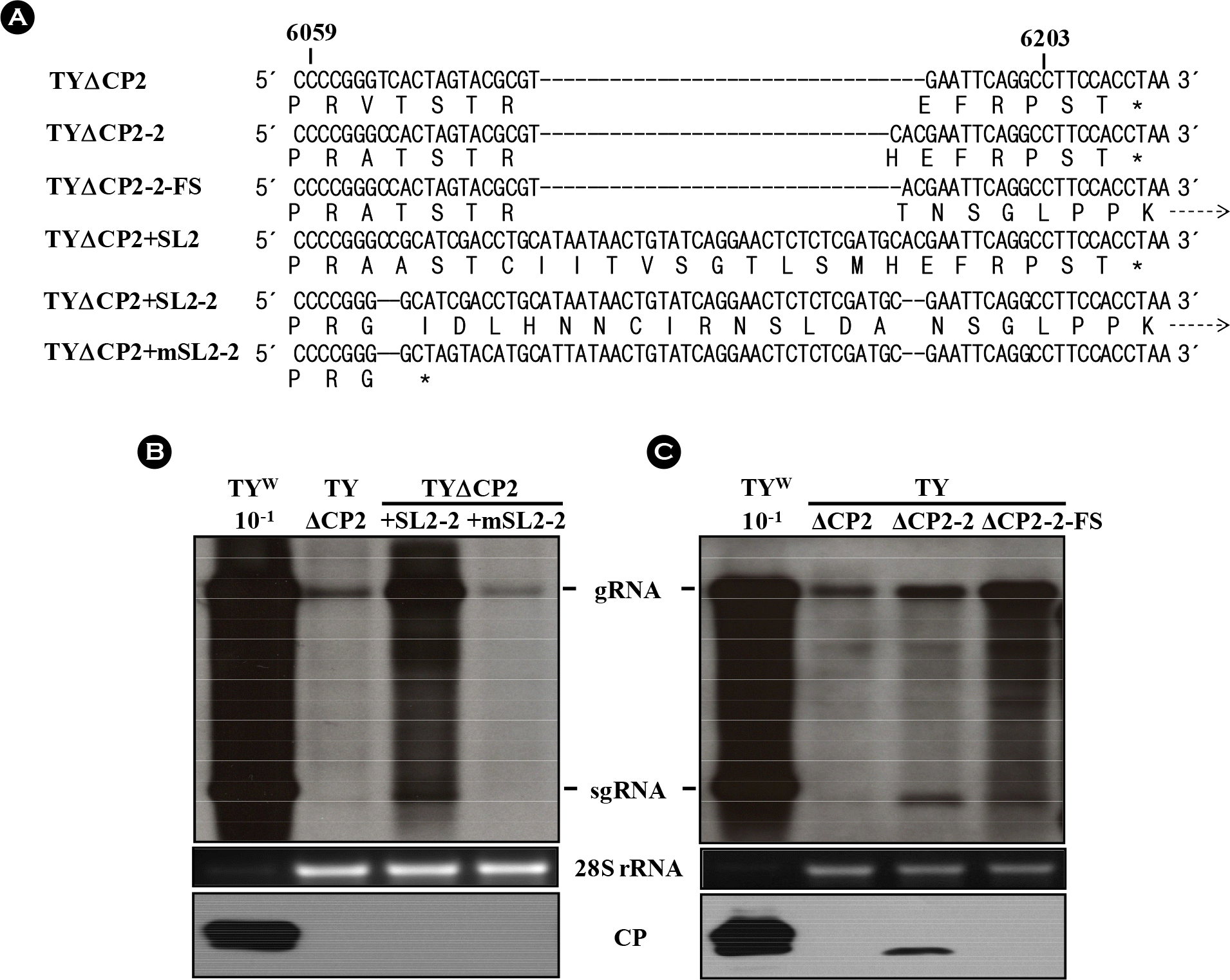 | Figure 6.Effect of read-through mutations on the replication of TYΔCP2 and TYΔCP2+SL2 variants. (A) Alignment of various TYΔCP2 and TYΔCP2+SL2 variants and their expected translation products. TYΔCP2-2-FS has one nucleotide deletion, compared to the TYΔCP2-2. This deletion results in frameshift and read-through beyond the CP ORF, as in TYΔCP2+m1′∼TYΔCP2+m4′ constructs. TYΔCP2+SL2-2 has a four-nucleotide (CC/AC) deletion, also resulting in read-through beyond the CP ORF. In TYΔCP2+mSL2-2, the four nucleotide (CC/AC) deletion leads to premature termination. Broken arrows indicate read-through. (B) Replication of TYΔCP2+ SL2-2 and TYΔCP2+mSL2-2 constructs. (C) Replication of TYΔCP2-2-FS. Total RNAs from the agroinfiltrated with the constructs were analyzed by Northern blot hybridization, as described in Fig. 2. Bottom panels represent the results of the western analysis of CP. |
Table 1.
Linkers used in this study




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download