Abstract
Vibrio vulnificus causes primary septicemia as a result of the consumption of contaminated seafood. The intestinal epithelial layer is the first host barrier encountered by V. vulnificus upon oral intake; however, epithelial translocation (invasion) of V.vulnificus has not been extensively studied. In this study, we investigated in vivo translocation of V. vulnificus using clinical (CMCP6) and environmental isolates (96-11-17M). And we analyzed physiological changes of intestinal epithelium concurrent with bacterial translocation by using polarized HCA-7 transwell culture system. The efficiency of epithelial translocation of 97-11-17M strains was significantly lower than that of pathogenic clinical isolate CMCP6 in a murine ligated ileal loop model. In an oral infection model, the survival rate was reciprocally related with efficacy of in vivo epithelial translocation. These results indicate that efficient translocation of V. vulnificus through intestinal epithelium is highly correlated with successful oral infection. We determined translocation of the bacteria from upper to lower chamber, changes of transepithelial electric resistance (TER) and cytotoxicity of the polarized HCA-7 cells to understand general features of V. vulnificus invasion. Bacterial translocation was accompanied by big decrease of TER (about 90%) and about 50% cytotoxicity of the epithelial cells. Taken together, these results indicate that V. vulnificus actively translocates the epithelium by destruction of epithelium and the efficiency of intestinal invasion by V. vulnificus is critical for successful oral infection. From this result, it is suggested that integrity of intestinal barrier is an important factor for susceptibility to oral infection of V. vulnificus.
REFERENCES
1). Koenig KL, Mueller J, Rose T. Vibrio vulnificus. Hazard on the half shell. West J Med. 1991; 155:400–3.
2). Gulig PA, Bourdage KL, Starks AM. Molecular Pathogenesis of Vibrio vulnificus. J Microbiol. 2005; 43:118–31.
3). Ritchie JM, Waldor MK. Vibrio cholerae interactions with the gastrointestinal tract: lessons from animal studies. Curr Top Microbiol Immunol. 2009; 337:37–59.
4). Conner CP, Heithoff DM, Mahan MJ. In vivo gene expression: contributions to infection, virulence, and pathogenesis. Curr Top Microbiol Immunol. 1998; 225:1–12.
5). Kim YR, Kim BU, Kim SY, Kim CM, Na HS, Koh JT, et al. Outer membrane vesicles of Vibrio vulnificus deliver cytolysin-hemolysin VvhA into epithelial cells to induce cytotoxicity. Biochem Biophys Res Commun. 2010; 399:607–12.
6). Kim YR, Lee SE, Kim CM, Kim SY, Shin EK, Shin DH, et al. Characterization and pathogenic significance of Vibrio vulnificus antigens preferentially expressed in septicemic patients. Infect Immun. 2003; 71:5461–71.
7). Beltinger J, del Buono J, Skelly MM, Thornley J, Spiller RC, Stack WA, et al. Disruption of colonic barrier function and induction of mediator release by strains of Campylobacter jejuni that invade epithelial cells. World J Gastroenterol. 2008; 14:7345–52.
8). Zhu W, Phan QT, Boontheung P, Solis NV, Loo JA, Filler SG. EGFR and HER2 receptor kinase signaling mediate epithelial cell invasion by Candida albicans during oropharyngeal infection. Proc Natl Acad Sci U S A. 2012; 109:14194–9.
9). Hoy B, Löwer M, Weydig C, Carra G, Tegtmeyer N, Geppert T, et al. Helicobacter pylori HtrA is a new secreted virulence factor that cleaves E-cadherin to disrupt intercellular adhesion. EMBO Rep. 2010; 11:798–804.
10). Nazli A, Chan O, Dobson-Belaire WN, Ouellet M, Tremblay MJ, Gray-Owen SD, et al. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 2010; 6:e1000852.

11). Guttman JA, Finlay BB. Tight junctions as targets of infectious agents. Biochim Biophys Acta. 2009; 1788:832–41.

12). Berkes J, Viswanathan VK, Savkovic SD, Hecht G. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut. 2003; 52:439–51.

13). Katz J, Sambandam V, Wu JH, Michalek SM, Balkovetz DF. Characterization of Porphyromonas gingivalis-induced degradation of epithelial cell junctional complexes. Infect Immun. 2000; 68:1441–9.
14). Giri CP, Shima K, Tall BD, Curtis S, Sathyamoorthy V, Hanisch B, et al. Cronobacter spp. (previously Enterobacter sakazakii) invade and translocate across both cultured human intestinal epithelial cells and human brain microvascular endothelial cells. Microb Pathog. 2012; 52:140–7.
15). Brás AM, Ketley JM. Transcellular translocation of Campylobacter jejuni across human polarised epithelial monolayers. FEMS Microbiol Lett. 1999; 179:209–15.
16). Steukers L, Glorieux S, Vandekerckhove AP, Favoreel HW, Nauwynck HJ. Diverse microbial interactions with the basement membrane barrier. Trends Microbiol. 2012; 20:147–55.

17). Kim HU, Kim SY, Jeong H, Kim TY, Kim JJ, Choy HE, et al. Integrative genome-scale metabolic analysis of Vibrio vulnificus for drug targeting and discovery. Mol Syst Biol. 2011; 7:460.
18). Høi L, Larsen JL, Dalsgaard I, Dalsgaard A. Occurrence of Vibrio vulnificus biotypes in Danish marine environments. Appl Environ Microbiol. 1998; 64:7–13.
19). Kim YR, Lee SE, Kook H, Yeom JA, Na HS, Kim SY, et al. Vibrio vulnificus RTX toxin kills host cells only after contact of the bacteria with host cells. Cell Microbiol. 2008; 10:848–62.
20). Lee SE, Kim SY, Kim CM, Kim MK, Kim YR, Jeong K, et al. The pyrH gene of Vibrio vulnificus is an essential in vivo survival factor. Infect Immun. 2007; 75:2795–801.
21). Lee SE, Song JA. Correlation among various virulence factors of Vibrio vulnificus. Chonnam Med J. 2002; 38:210–7.
22). Reed LJ, Muench H. A simple method of estimating the fifty percent end points. Am J Hyg. 1938; 27:493–7.
23). Lee J, Mo JH, Katakura K, Alkalay I, Rucker AN, Liu YT, et al. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol. 2006; 8:1327–36.

24). Poole MD, Oliver JD. Experimental pathogenicity and mortality in ligated ileal loop studies of the newly reported halophilic lactose-positive Vibrio sp. Infect Immun. 1978; 20:126–9.
25). Morrison SS, Williams T, Cain A, Froelich B, Taylor C, Baker-Austin C, et al. Pyrosequencing-based comparative genome analysis of Vibrio vulnificus environmental isolates. PLoS One. 2012; 7:e37553.
26). Shinoda S, Kobayashi M, Yamada H, Yoshida S, Ogawa M, Mizuguchi Y. Inhibitory effect of capsular antigen of Vibrio vulnificus on bactericidal activity of human serum. Microbiol Immunol. 1987; 31:393–401.
27). Strom MS, Paranjpye RN. Epidemiology and pathogenesis of Vibrio vulnificus. Microbes Infect. 2000; 2:177–88.
28). Musher DM, Hansen MV, Goree A, Gyorkey F, Chapman AJ, Baughn RE. Emergence of bactericidal and opsonizing antibody to Vibrio vulnificus following bacterial infection. J Clin Microbiol. 1986; 23:411–5.
29). Jones MK, Oliver JD. Vibrio vulnificus: disease and pathogenesis. Infect Immun. 2009; 77:1723–33.
30). Mabbott NA, Donaldson DS, Ohno H, Williams IR, Mahajan A. Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 2013; 6:666–77.

Figure 1.
In vivo invasion by V. vulnificus strains in a ligated ileal loop infection model. V. vulnificus (4.0 × 106 CFU/400 μl) was inoculated into ligated ileal loops of mice under anesthesia. After 3 and 6 hours, blood samples were collected from the ocular plexus from the infected mice. Viable bacterial cells were counted by plating on 2.5% NaCl HI agar plates. Bacterial invasion from the ligated ileal loop into bloodstream was significantly decreased in 96-11-17M infected mice. The data represent the average values and SEM from three or four mice. *p < 0.05; **p < 0.01 compared with V. vulnificus CMCP6.
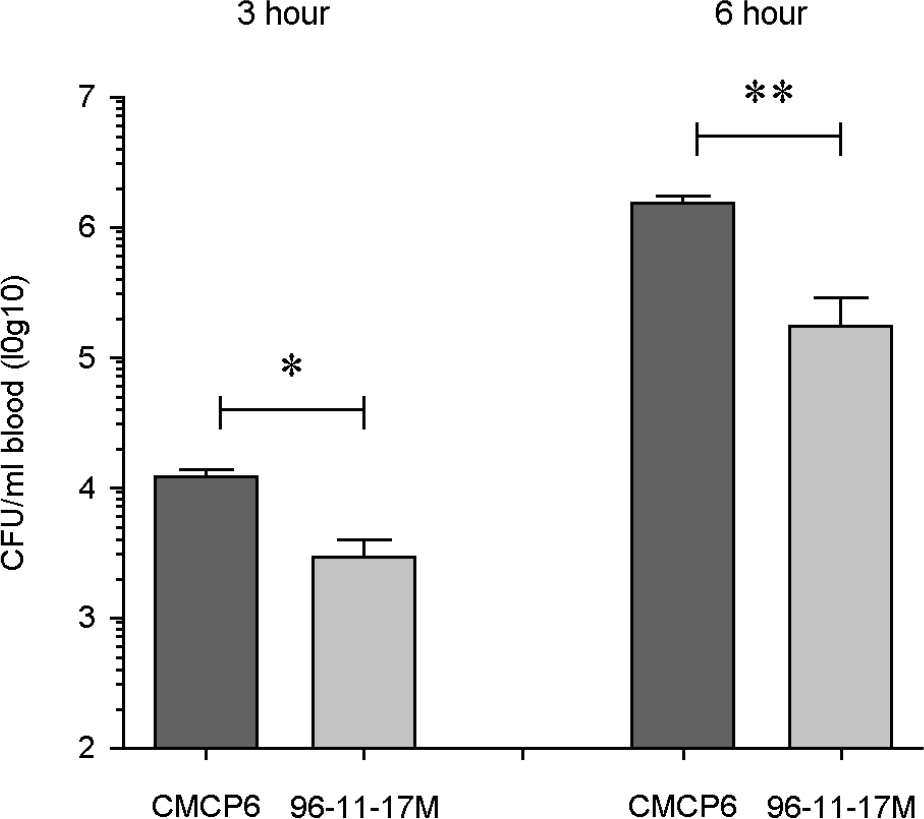
Figure 2.
Survival curve for mice infected intragastrically with V. vulnificus strains at a dose of 1.0 × 109 cfu/mouse (A) or 1.0 × 108 cfu/mouse (B). The survival rate was significantly increased in the 96-11-17M-infected mice. The survival curve was constructed according to the Kaplan-Meier method, and statistical significance was determined by the log-rank test. *p < 0.05; **p < 0.01 compared with V. vulnificus CMCP6.
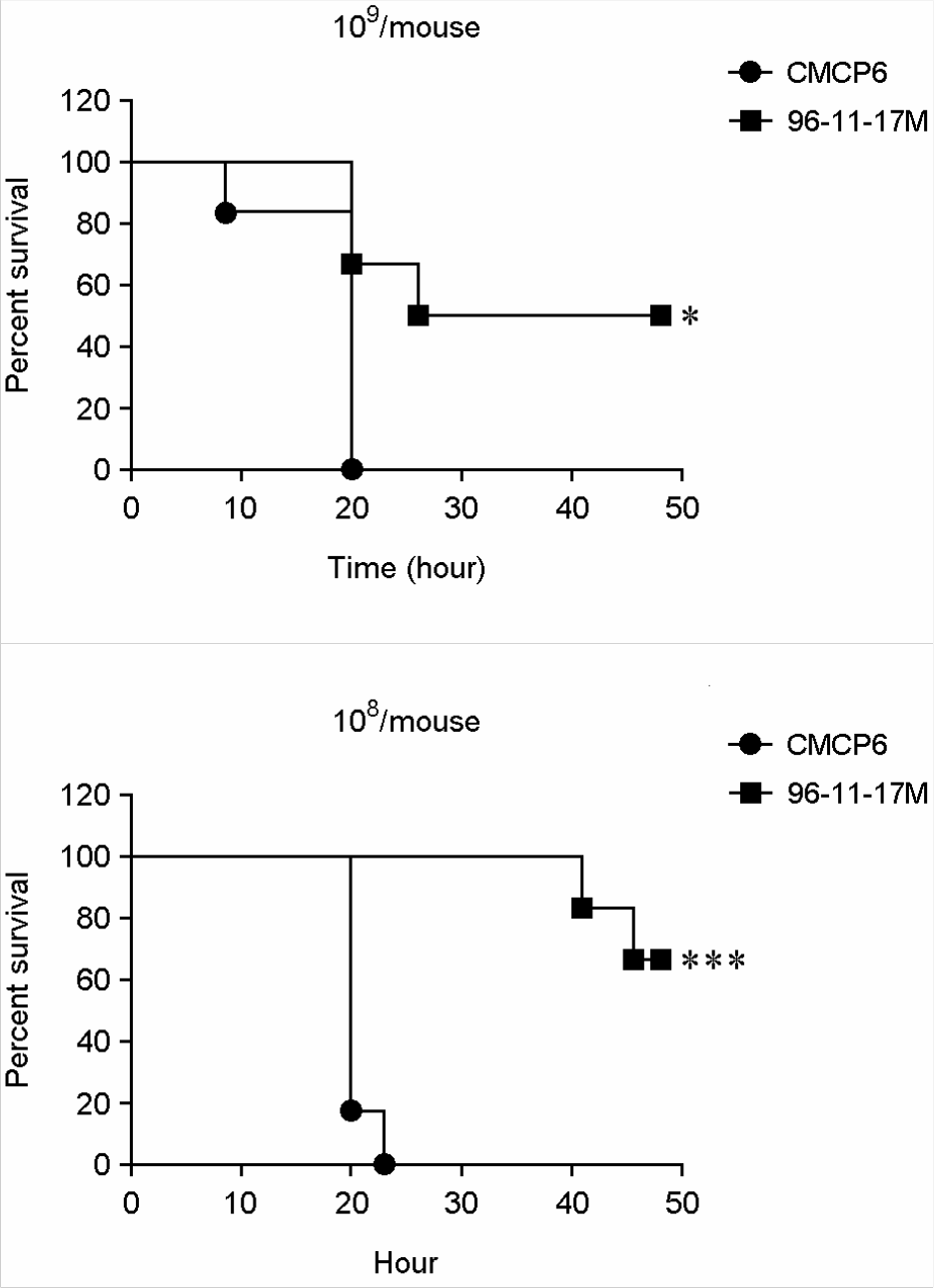
Figure 3.
In vitro invasion by V. vulnificus CMCP6 and 96-11-17M in polarized HCA-7 cell monolayers. HCA-7 cells grown on transwell filters were apically infected with V. vulnificus CMCP6 or 96-11-17M. The bacterial epithelial translocation (A) was determined by measuring the number of bacterial cells that translocated from the upper chamber to the lower chamber of the transwell. The change in transepithelial electrical resistance (TER) (B) and LDH release in the upper (apical) and lower chamber (basolateral) (C) were determined. Viable bacterial cells were counted by plating on 2.5% NaCl HI agar plates.
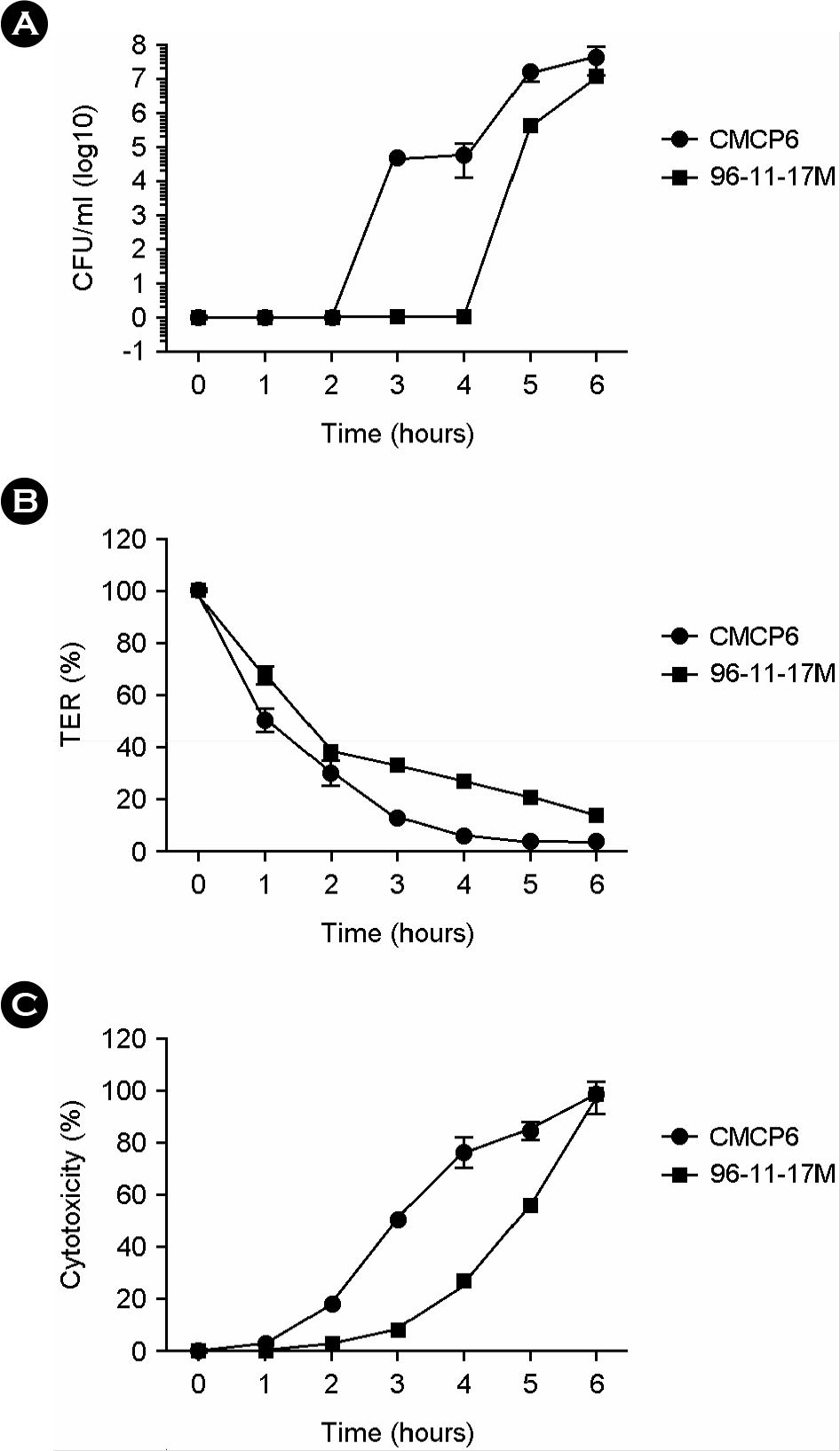
Table 1.
Bacterial strains used in this study
Table 2.
Generation time of V. vulnificus in dialysis tubing implantation model
| Strains | Generation time (min)a | |
|---|---|---|
| Mean ± SEM | P value | |
| CMCP6 | 22.41 ± 0.62 | p > 0.05 |
| 96-11-17M | 23.29 ± 1.44 | p > 0.05 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download