Abstract
Viruses interact with the host ubiquitination system in a variety of ways. Viral proteins are often a substrate for ubiquitination, which leads to proteasomal degradation. Viruses also have functions to modify the cellular ubiquitination machinery. Recently, deubiquitinating protease (DUB) activity has been found in many viral proteins. In herpesviruses, the DUB domain is found within the large tegument protein, which is conserved in all members of the herpesvirus family. Although a limited number of viral and cellular targets have been identified to date, accumulating evidence shows that herpesviral DUBs may primarily target key cellular regulators of immune signaling pathways to promote viral replication. In this review, we summarize the recent findings on viral DUBs. In particular, we focus on the herpesviral DUBs and their targets, and discuss their potential roles in the regulation of immune signaling pathways.
Go to : 
REFERENCES
3). Glickman MH, Ciechanover A. The ubiquitinproteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002; 82:373–428.

4). Schnell JD, Hicke L. Non-traditional functions of ubiquitin and ubiquitin-binding proteins. J Biol Chem. 2003; 278:35857–60.

5). Paludan SR, Bowie AG, Horan KA, Fitzgerald KA. Recognition of herpesviruses by the innate immune system. Nat Rev Immunol. 2011; 11:143–54.

6). Vucic D, Dixit VM, Wertz IE. Ubiquitylation in apoptosis: a post-translational modification at the edge of life and death. Nat Rev Mol Cell Biol. 2011; 12:439–52.

8). Schlieker C, Weihofen WA, Frijns E, Kattenhorn LM, Gaudet R, Ploegh HL. Structure of a herpesvirus-encoded cysteine protease reveals a unique class of deubiquitinating enzymes. Mol Cell. 2007; 25:677–87.

9). Isaacson MK, Ploegh HL. Ubiquitination, ubiquitin-like modifiers, and deubiquitination in viral infection. Cell Host Microbe. 2009; 5:559–70.

10). Balakirev MY, Jaquinod M, Haas AL, Chroboczek J. Deubiquitinating function of adenovirus proteinase. J Virol. 2002; 76:6323–31.

11). Lindner HA, Fotouhi-Ardakani N, Lytvyn V, Lachance P, Sulea T, Ménard R. The papain-like protease from the severe acute respiratory syndrome coronavirus is a deubiquitinating enzyme. J Virol. 2005; 79:15199–208.

12). Frias-Staheli N, Giannakopoulos NV, Kikkert M, Taylor SL, Bridgen A, Paragas J, et al. Ovarian tumor domain-containing viral proteases evade ubiquitin- and ISG15-dependent innate immune responses. Cell Host Microbe. 2007; 2:404–16.

13). Kattenhorn LM, Korbel GA, Kessler BM, Spooner E, Ploegh HL. A deubiquitinating enzyme encoded by HSV-1 belongs to a family of cysteine proteases that is conserved across the family Herpesviridae. Mol Cell. 2005; 19:547–57.

14). Schlieker C, Korbel GA, Kattenhorn LM, Ploegh HL. A deubiquitinating activity is conserved in the large tegument protein of the herpesviridae. J Virol. 2005; 79:15582–5.
15). Wang J, Loveland AN, Kattenhorn LM, Ploegh HL, Gibson W. High-molecular-weight protein (pUL48) of human cytomegalovirus is a competent deubiquitinating protease: mutant viruses altered in its active-site cysteine or histidine are viable. J Virol. 2006; 80:6003–12.

16). Jarosinski K, Kattenhorn L, Kaufer B, Ploegh H, Osterrieder N. A herpesvirus ubiquitin-specific protease is critical for efficient T cell lymphoma formation. Proc Natl Acad Sci U S A. 2007; 104:20025–30.

17). Böttcher S, Maresch C, Granzow H, Klupp BG, Teifke JP, Mettenleiter TC. Mutagenesis of the active-site cysteine in the ubiquitin-specific protease contained in large tegument protein pUL36 of pseudorabies virus impairs viral replication in vitro and neuroinvasion in vivo. J Virol. 2008; 82:6009–16.
18). Sompallae R, Gastaldello S, Hildebrand S, Zinin N, Hassink G, Lindsten K, et al. Epstein-barr virus encodes three bona fide ubiquitin-specific proteases. J Virol. 2008; 82:10477–86.

19). González CM, Wang L, Damania B. Kaposi's sarcoma-associated herpesvirus encodes a viral deubiquitinase. J Virol. 2009; 83:10224–33.

20). Gredmark-Russ S, Isaacson MK, Kattenhorn L, Cheung EJ, Watson N, Ploegh HL. A gammaherpesvirus ubiquitin-specific protease is involved in the establishment of murine gammaherpesvirus 68 infection. J Virol. 2009; 83:10644–52.

21). Roberts AP, Abaitua F, O'Hare P, McNab D, Rixon FJ, Pasdeloup D. Differing roles of inner tegument proteins pUL36 and pUL37 during entry of herpes simplex virus type 1. J Virol. 2009; 83:105–16.

22). Shanda SK, Wilson DW. UL36p is required for efficient transport of membrane-associated herpes simplex virus type 1 along microtubules. J Virol. 2008; 82:7388–94.

23). Abaitua F, O'Hare P. Identification of a highly conserved, functional nuclear localization signal within the N-terminal region of herpes simplex virus type 1 VP1-2 tegument protein. J Virol. 2008; 82:5234–44.

24). Jovasevic V, Liang L, Roizman B. Proteolytic cleavage of VP1-2 is required for release of herpes simplex virus 1 DNA into the nucleus. J Virol. 2008; 82:3311–9.

25). Morrison EE, Stevenson AJ, Wang YF, Meredith DM. Differences in the intracellular localization and fate of herpes simplex virus tegument proteins early in the infection of Vero cells. J Gen Virol. 1998; 79:2517–28.

26). Desai PJ. A null mutation in the UL36 gene of herpes simplex virus type 1 results in accumulation of unenveloped DNA-filled capsids in the cytoplasm of infected cells. J Virol. 2000; 74:11608–18.

27). Gack MU, Shin YC, Joo CH, Urano T, Liang C, Sun L, et al. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature. 2007; 446:916–20.

28). Oshiumi H, Matsumoto M, Hatakeyama S, Seya T. Riplet/RNF135, a RING finger protein, ubiquitinates RIG-I to promote interferon-beta induction during the early phase of viral infection. J Biol Chem. 2009; 284:807–17.
29). Häcker H, Redecke V, Blagoev B, Kratchmarova I, Hsu LC, Wang GG, et al. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature. 2006; 439:204–7.

30). Kayagaki N, Phung Q, Chan S, Chaudhari R, Quan C, O'Rourke KM, et al. DUBA: a deubiquitinase that regulates type I interferon production. Science. 2007; 318:1628–32.
31). Oganesyan G, Saha SK, Guo B, He JQ, Shahangian A, Zarnegar B, et al. Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature. 2006; 439:208–11.

32). Saha SK, Pietras EM, He JQ, Kang JR, Liu SY, Oganesyan G, et al. Regulation of antiviral responses by a direct and specific interaction between TRAF3 and Cardif. EMBO J. 2006; 25:3257–63.

33). Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005; 122:669–82.
34). Wang S, Wang K, Li J, Zheng C. Herpes Simplex Virus 1 Ubiquitin-Specific Protease UL36 Inhibits Beta Interferon Production by Deubiquitinating TRAF3. J Virol. 2013; 87:11851–60.

35). Kim ET, Oh SE, Lee YO, Gibson W, Ahn JH. Cleavage specificity of the UL48 deubiquitinating protease activity of human cytomegalovirus and the growth of an activesite mutant virus in cultured cells. J Virol. 2009; 83:12046–56.

36). Sun SC. Deubiquitylation and regulation of the immune response. Nat Rev Immunol. 2008; 8:501–11.

37). Whitehurst CB, Ning S, Bentz GL, Dufour F, Gershburg E, Shackelford J, et al. The Epstein-Barr virus (EBV) deubiquitinating enzyme BPLF1 reduces EBV ribonucleotide reductase activity. J Virol. 2009; 83:4345–53.

38). Ernst R, Claessen JH, Mueller B, Sanyal S, Spooner E, van der Veen AG, et al. Enzymatic blockade of the ubiquitin-proteasome pathway. PLoS Biol. 2011; 8:e1000605.

39). Whitehurst CB, Vaziri C, Shackelford J, Pagano JS. Epstein-Barr virus BPLF1 deubiquitinates PCNA and attenuates polymerase eta recruitment to DNA damage sites. J Virol. 2012; 86:8097–106.
40). Gastaldello S, Hildebrand S, Faridani O, Callegari S, Palmkvist M, Di Guglielmo C, et al. A deneddylase encoded by Epstein-Barr virus promotes viral DNA replication by regulating the activity of cullin-RING ligases. Nat Cell Biol. 2010; 12:351–61.

41). Gastaldello S, Chen X, Callegari S, Masucci MG. Caspase-1 promotes epstein-barr virus replication by targeting the large tegument protein deneddylase to the nucleus of productively infected cells. PLoS Pathog. 2013; 9:e1003664.

42). Saito S, Murata T, Kanda T, Isomura H, Narita Y, Sugimoto A, et al. Epstein-Barr virus deubiquitinase downregulates TRAF6-mediated NF-kappaB signaling during productive replication. J Virol. 2013; 87:4060–70.
43). Song YJ. ASK1 is involved in EBV LMP1-induced NF-κB activation. J Bacteriol Virol. 2012; 42:63–8.

44). Arcipowski KM, Stunz LL, Graham JP, Kraus ZJ, Vanden Bush TJ, Bishop GA. Molecular mechanisms of TNFR-associated factor 6 (TRAF6) utilization by the oncogenic viral mimic of CD40, latent membrane protein 1 (LMP1). J Biol Chem. 2011; 286:9948–55.

45). Inn KS, Lee SH, Rathbun JY, Wong LY, Toth Z, Machida K, et al. Inhibition of RIG-I-mediated signaling by Kaposi's sarcoma-associated herpesvirus-encoded deubiquitinase ORF64. J Virol. 2011; 85:10899–904.

46). Arimoto K, Takahashi H, Hishiki T, Konishi H, Fujita T, Shimotohno K. Negative regulation of the RIG-I signaling by the ubiquitin ligase RNF125. Proc Natl Acad Sci U S A. 2007; 104:7500–5.

47). Fuchs W, Klupp BG, Granzow H, Mettenleiter TC. Essential function of the pseudorabies virus UL36 gene product is independent of its interaction with the UL37 protein. J Virol. 2004; 78:11879–89.

48). Luxton GW, Lee JI, Haverlock-Moyns S, Schober JM, Smith GA. The pseudorabies virus VP1/2 tegument protein is required for intracellular capsid transport. J Virol. 2006; 80:201–9.

49). Zaichick SV, Bohannon KP, Hughes A, Sollars PJ, Pickard GE, Smith GA. The herpesvirus VP1/2 protein is an effector of dynein-mediated capsid transport and neuroinvasion. Cell Host Microbe. 2013; 13:193–203.

Go to : 
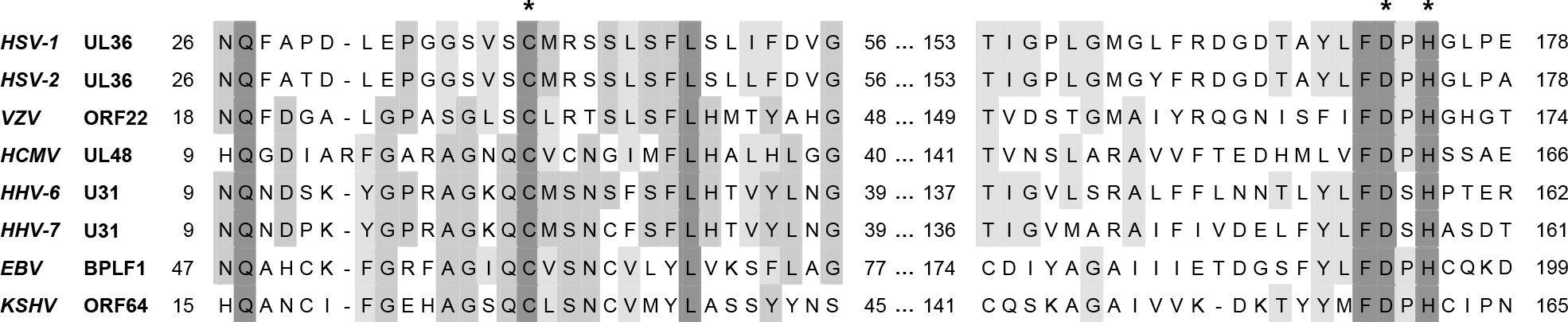 | Figure 1.
Amino acid alignment of the conserved catalytic active site region of the DUB domains from human herpesviruses. Amino acids in the conserved region surrounding the active site cysteine residue in the DUBs of human herpesviruses are aligned. Black boxes indicate conserved residues, and grey boxes indicate similar residues. The cysteine, aspartic acid, and histidine residues in the catalytic triad are indicated by asterisks. |
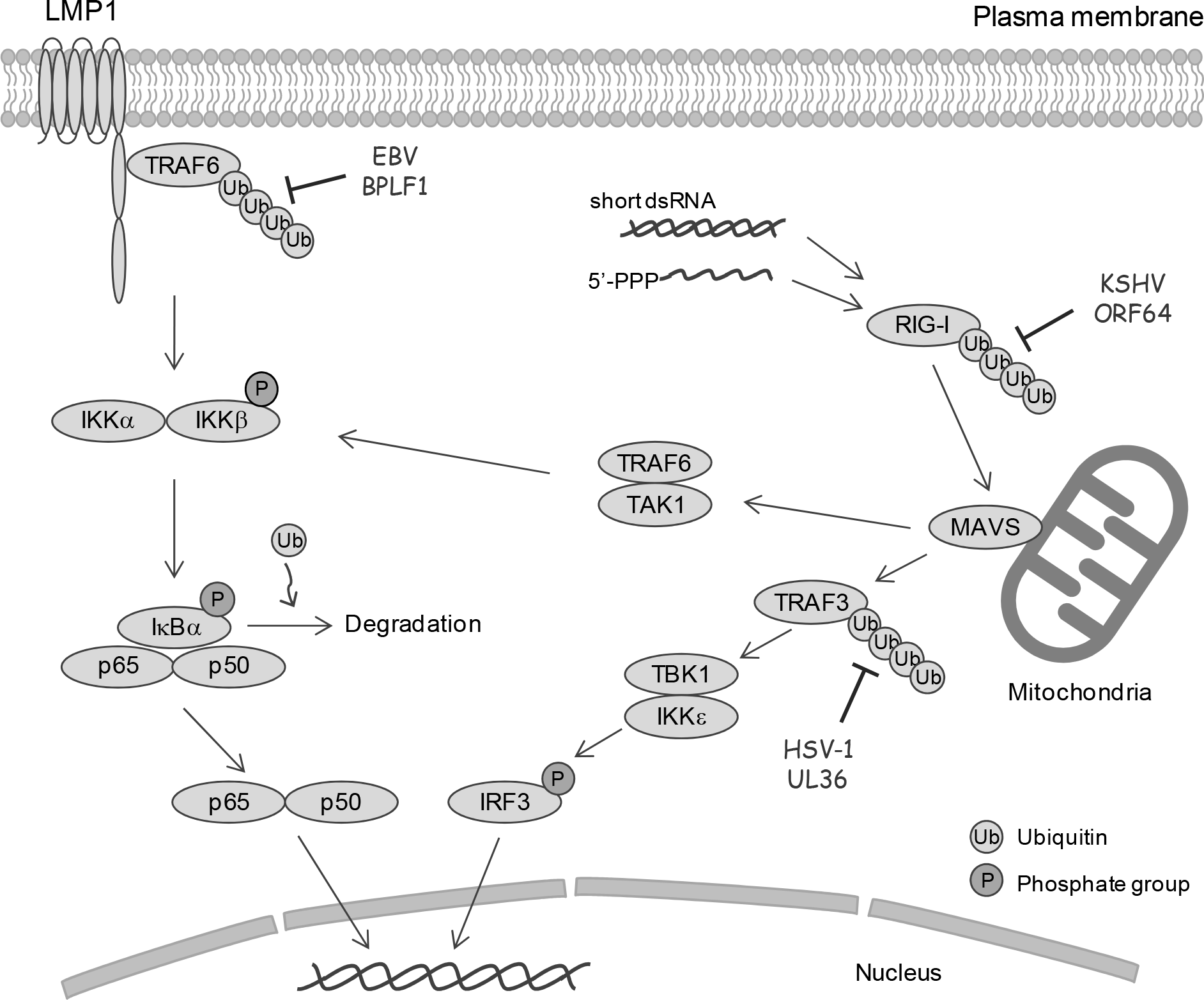 | Figure 2.
The NF-κB and IRF3 signaling pathways and the targets of herpesvirus DUBs. In EBV latent infection, NF-κB is activated by viral LMP1. TRAF6 associates with LMP1 and is constitutively polyubiquitinated. Activation of NF-κB confers cell survival and inhibits the spontaneous induction of lytic replication. Once lytic replication is induced, BPLF1 deubiquitinates and inactivates TRAF6 to block NF-κB signaling, promoting efficient viral genome replication. RIG-I is a cytosolic RNA sensor that recognizes viral RNA. Ubiquitination of RIG-I promotes its association with MAVS, and ubiquitination of TRAF3 mediates recruitment of the TBK1/IKK∊ complex, leading to activation of IRF3. KSHV ORF64 and HSV-1 UL36 deubiquitinate RIG-I and TRAF3, respectively, thus inhibiting the RIG-mediated activation of IRF3 and type I IFN production. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download