Abstract
The purpose of this study was to explore the laboratory biosafety status of Public Health Centers (PHCs) in Korea during Oct.7∼26, 2012. We surveyed the environment of biosafety management, especially for the recognition level for biosafety of workers in the organizations. The questionnaires given out to 98 workers who are working for PHCs are to research the recognition level of workers for the knowledge of biosafety, related laws and regulations. The level was the highest in the Research Institute of the Public Health & Environment (RIPHE) followed by quarantine station, and the health center was assessed as the last. It was turned out that the biosafety educational program in the RIPHE was implemented on a regular basis (65.2%) with irregular cases (21.7%), and some outsourcing chances (8.7%). However, quarantine stations and health centers didn't practice actively biosafety training programs compared to RIPHE. In addition, there was a majority of opinions that the most important thing to improve biosafety level of PHCs is to strengthen current poor training and education system. In conclusion, it is necessary to develop more improved training system for biosafety on exposure risks including injuries, personal protective equipment, and chemical hazards.
REFERENCES
1). Shaw K. The 2003 SARS outbreak and its impact on infection control practices. Public Health. 2006; 120:8–14.

2). Zhao Z, Zhang F, Xu M, Huang K, Zhong W, Cai W, et al. Description and clinical treatment of an early outbreak of severe acute respiratory syndrome (SARS) in Guangzhou, PR China. J Med Microbiol. 2003; 52:715–20.

3). Gatherer D. The 2009 H1N1 influenza outbreak in its historical context. J Clin Virol. 2009; 45:174–8.

4). Tellier R. Aerosol transmission of influenza A virus: a review of new studies. J R Soc Interface. 2009; 6:783–90.

5). Summary of human infection with highly pathogenic avian influenza A (H5N1) virus reported to WHO, January 2003-March 2009 cluster associated cases. Wkly Epidemiol Rec. 2010; 85:13–20.
7). Inglesby TV, O'Toole T, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, et al. Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA. 2002; 287:2236–52.
8). Henderson DA, Inglesby TV, Bartlett JG, Ascher MS, Eitzen E, Jahrling PB, et al. Smallpox as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. JAMA. 1999; 281:2127–37.
9). Jank B, Gaugitsch H. Decision making under the Cartagena Protocol on Biosafety. Trends Biotechnol. 2001; 19:194–7.

10). Lee JY, Eun SJ, Park KD, Kim JK, Im JS, Hwang YS, et al. Biosafety of microbiological laboratories in Korea. J Prev Med Public Health. 2005; 38:449–56.
11). Martini GA, Schmidt HA. Spermatogenic transmission of the “Marburg virus”. Klin Wochenschr. 1968; 46:398–400.
12). Oliphant JW, Parker RR. Q fever: Three cases of laboratory infection. Public Health Rep. 1948; 63:1364–70.

14). Holmes GP, Hilliard JK, Klontz KC, Rupert AH, Schindler CM, Parrish E, et al. B virus (Herpesvirus simiae) infection in humans: epidemiologic investigation of a cluster. Ann Intern Med. 1990; 112:833–9.

15). Barton Behravesh C, Mody RK, Jungk J, Gaul L, Redd JT, Chen S, et al. 2008 outbreak of Salmonella Saintpaul infections associated with raw produce. N Engl J Med. 2011; 364:918–27.

16). Cho A, Seok SH. Ethical Guidelines for Use of Experimental Animals in Biomedical Research. J Bacteriol Virol. 2013; 43:18–26.

17). Barkham TM. Laboratory safety aspects of SARS at Biosafety Level 2. Ann Acad Med Singapore. 2004; 33:252–6.
18). Lim W, Ng KC, Tsang DN. Laboratory containment of SARS virus. Ann Acad Med Singapore. 2006; 35:354–60.
19). MacNeil A, Reynolds MG, Damon IK. Risks associated with vaccinia virus in the laboratory. Virology. 2009; 385:1–4.

20). Chong PY, Chui P, Ling AE, Franks TJ, Tai DY, Leo YS, et al. Analysis of deaths during the severe acute respiratory syndrome (SARS) epidemic in Singapore: challenges in determining a SARS diagnosis. Arch Pathol Lab Med. 2004; 128:195–204.

21). Koh D, Sng J. Lessons from the past: perspectives on severe acute respiratory syndrome. Asia Pac J Public Health. 2010; 22:132S–6S.

22). Orellana C. Laboratory-acquired SARS raises worries on biosafety. Lancet Infect Dis. 2004; 4:64.

23). Jiang TJ, Zhou XZ, Zhao M, Zhou ZP, Jiang SC, Ye WH, et al. Analysis of severe acute respiratory syndrome in Beijing. Zhonghua Nei Ke Za Zhi. 2003; 42:369–72.
24). Lee HW, Johnson KM. Laboratory-acquired infections with hantaan virus, the etiologic agent of Korean hemorrhagic fever. J Infec Dis. 1982; 146:645–51.

25). Kim JS, Lee HW. Studies on microbiololgical laboratory biosafety a survey on biosafety status in laboratory department of training. Report NIH Korea. 1986; 23:139–60.
26). Cho SH, Ju YS, Kang D, KiM S, Kim IS, Hong ST. Laboratory-acquired infection with hantavirus at a research unit of medical school in Seoul, 1996. Korean J Prev Med. 1999; 32:269–75.
27). U.S. Department of Health & Human Services, Center for Disease Control and Prevention, National Institutes of Health. Biosafety in Microbiological and Biomedical Laboratories (BMBL). 5th ed.Washington D.C.: CDC/NIH;2009. Available from: URL:. http://www.cdc.gov/biosafety/publications/bmbl5/BMBL.pdf.
28). World Health Organization. Laboratory biosafety manual. 3rd ed.Geneva: World Health Organization;2004. Available from: URL:. http://www.who.int/csr/resources/publications/biosafety/Biosafety7.pdf.
29). Hui Z, Jian-Shi H, Xiong H, Peng L, Da-Long Q. An analysis of the current status of hospital emergency preparedness for infectious disease outbreaks in Beijing, China. Am J Infect Control. 2007; 35:62–7.
Figure 1.
The routine inspection and disinfection check of the Laboratory equipment (N=98, %). The survey result on the routine inspection and disinfection check of the laboratory equipment. This research builds on previous study.
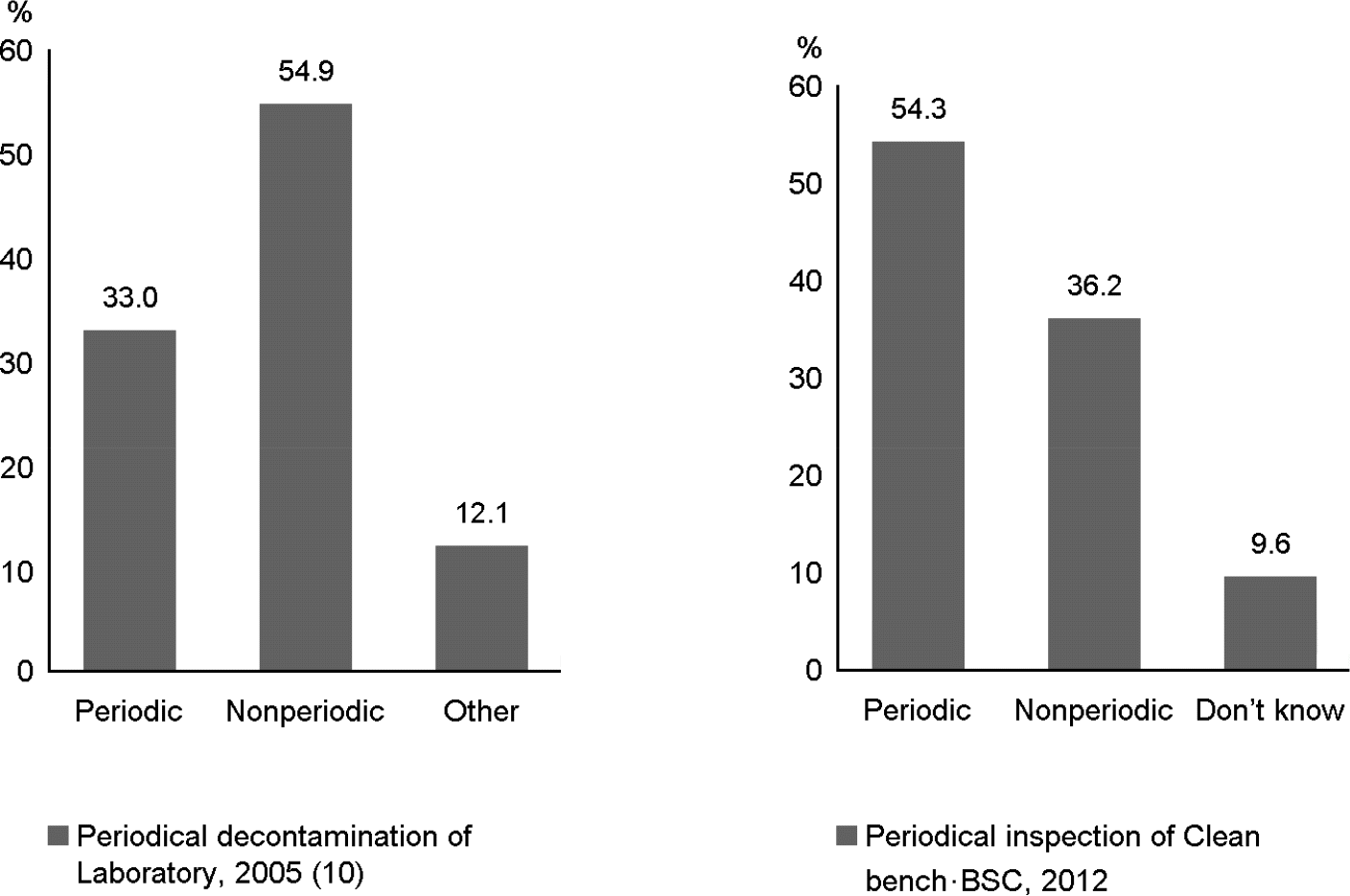
Figure 2.
Holding the Personal protective Equipment, 2005 (N=98, %) (10). Filed bars represent the rate of holding the personal protective equipment in 2005, respectively.
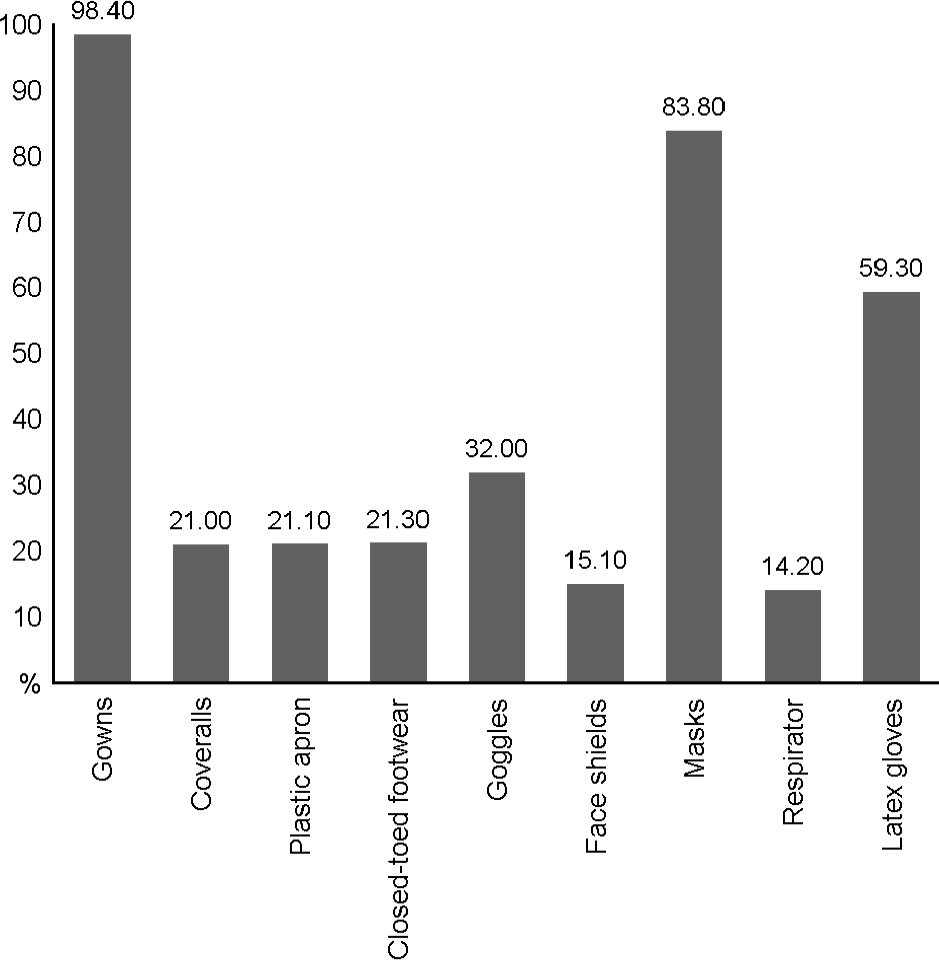
Figure 3.
The rate of use of Personal protective Equipment, 2012 (N=98, %). Filed bars represent the rate of use of personal protective equipment in 2012, respectively.
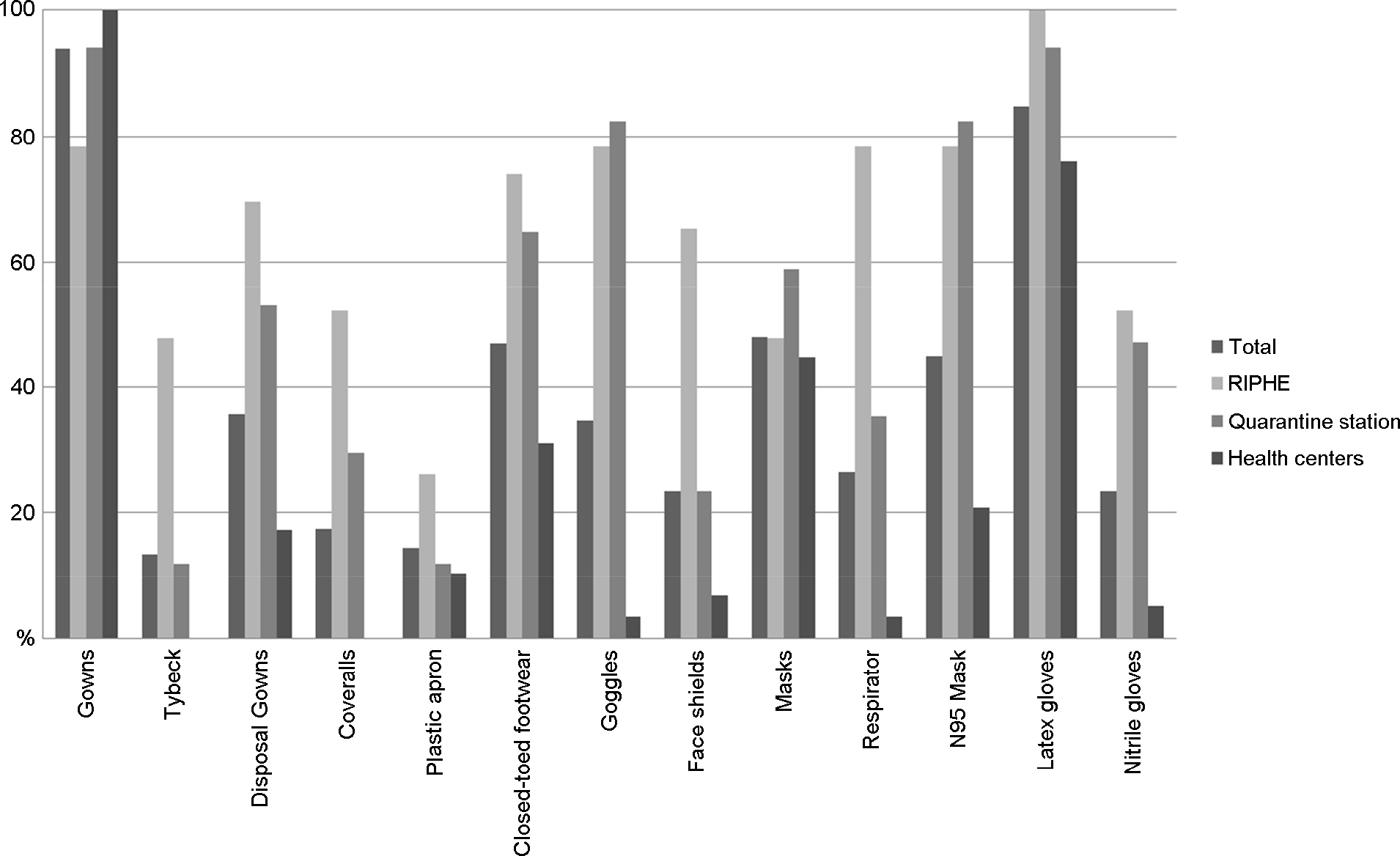
Figure 4.
Requirements for strengthen the laboratory biosafety level (N=98, %). The survey result showed that requirements for strengthen the laboratory biosafety
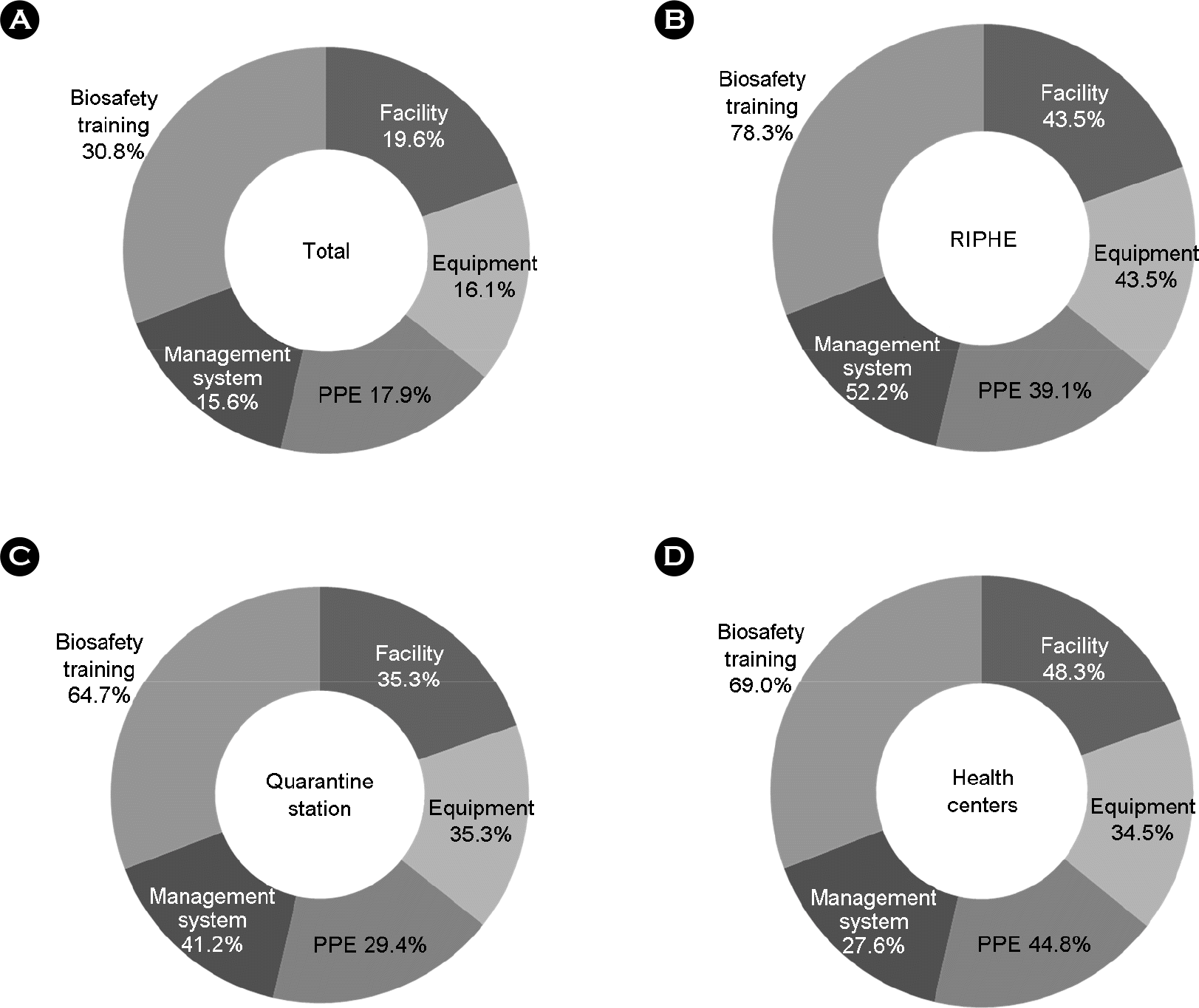
Table 1.
Biosafety level, BL (28)
| Risk level | Biosafety level | Laboratory practices | Safety equipment |
|---|---|---|---|
| 1 | BL1 | Basic laboratory | Open bench |
| 2 | BL2 | BL1 + Protective clothing, Biohazard Sign | Open bench + BSCa |
| 3 | BL3 | BL2 + Special clothing, Controlled access, Directional airflow | BSC + and/or other primary devices for all actives |
| 4 | BL4 | BL3 + Air lock, Shower exit, Special Waste disposal | Class III BSC, positive pressure suits, double-ended autoclave, filtered air |
Table 2.
The biosafety items used for estimating biosafety awareness
| Categories | Items |
|---|---|
| Required Biosafety level |
Risk group of pathogen (Bacteria, Virus, clinical specimen, etc) Biosafety level: BL1, 2, 3 |
| Infectious agents | Culture, Diagnostics, Storage, Transportation. |
| Biosafety management of laboratory |
Biosafety committee, Biosafety officer Risk assessment |
| Biosafety basic rules |
Responsibility for laboratory biosafety Biosafety, Biosecurity, Facility, Equipment, PPEa, Risk, HDPsb |
| Equipment for biosafety |
Required equipment for biosafety Appropriately used equipment |
| Personal protective equipment |
PPE-Research Requirements Sufficient supplies of PPE Availability and condition of lab equipment |
| Biosafety training |
Related laws and regulations BL3 facility (Design/Management/Equipment/PPE/HDPs) |
| Improving laboratory biosafety | Facility, Equipment, Biosafety management system, Provision of Safety Training |
Table 3.
The general characteristics of the survey respondents
Table 4.
Biosafety Management of public healthcare centers [Total n=98, n (%)]
Table 5.
An awareness and compliance of biosafety in Laboratories (N=98, %)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download