Abstract
The structure of coxsackievirus and adenovirus receptor's CAR is similar to adhesion molecules. In the adult heart, the majority of CAR localizes at the intercalated disc. Germ line CAR deletion induces embryonic lethality at E11.5 with evidence of a cardiac abnormality. The CAR role as a viral receptor is well known; however, its precise function in the heart for enterovirus infection is not clear. To understand the role of CAR in the cardiac myocyte, we generated cardiac-specific CAR knockout mice using a CAR floxed allele and alpha-MHC-Mer CRE Mer mice. Western blot analysis and immunofluorescent stain of ventricles at 6 weeks after 2 weeks tamoxifen administration, CAR expression was significantly decreased in CARf/f MCM mice but not in CARf/f mice heart. Enterovirus was intraperitoneally infected into CARf/f MCM and CARf/f mice (n=10 each). CAR disruption was dramatically reduced virus infection and replication in the heart but not different in liver, spleen, and pancreas. Cardiac myocyte damage was significantly reduced in the CARf/f MCM mutant mice by evans blue dye stain. In addition, the CARf/f MCM mutant mice heart inflammation and fibrosis were decreased in H&E and trichrome stain compare to CARf/f control mice. CAR expression was required for normal ventricular function, but it is the cause of enterovirus infection. In the adult mice heart, CAR deletion was significantly reduced viral infection, proliferation, and myocarditis. These results suggested that CAR deletion could be useful therapeutic strategy to prevent viral myocarditis.
Go to : 
REFERENCES
1). Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997; 275:1320–3.

2). Lim BK, Xiong D, Dorner A, Youn TJ, Yung A, Liu TI, et al. Coxsackievirus and adenovirus receptor (CAR) mediates atrioventricular-node function and connexin 45 localization in the murine heart. J Clin Invest. 2008; 118:2758–70.

3). Baboonian C, Davies MJ, Booth JC, McKenna WJ. Coxsackie B viruses and human heart disease. Curr Top Microbiol Immunol. 1997; 223:31–52.

4). Lim BK, Choi JH, Nam JH, Gil CO, Shin JO, Yun SH, et al. Virus receptor trap neutralizes coxsackievirus in experimental murine viral myocarditis. Cardiovasc Res. 2006; 71:517–26.

5). Coyne CB, Bergelson JM. CAR: a virus receptor within the tight junction. Adv Drug Deliv Rev. 2005; 57:869–82.

6). Noutsias M, Fechner H, de Jonge H, Wang X, Dekkers D, Houtsmuller AB, et al. Human coxsackie-adenovirus receptor is colocalized with integrins alpha(v)beta(3) and alpha(v)beta(5) on the cardiomyocyte sarcolemma and upregulated in dilated cardiomyopathy: implications for cardiotropic viral infections. Circulation. 2001; 104:275–80.
7). Dorner AA, Wegmann F, Butz S, Wolburg-Buchholz K, Wolburg H, Mack A, et al. Coxsackievirus-adenovirus receptor (CAR) is essential for early embryonic cardiac development. J Cell Sci. 2005; 118:3509–21.

8). Asher DR, Cerny AM, Weiler SR, Horner JW, Keeler ML, Neptune MA, et al. Coxsackievirus and adenovirus receptor is essential for cardiomyocyte development. Genesis. 2005; 42:77–85.

9). Kumai M, Nishii K, Nakamura K, Takeda N, Suzuki M, Shibata Y. Loss of connexin45 causes a cushion defect in early cardiogenesis. Development. 2000; 127:3501–12.

10). Egashira K, Nishii K, Nakamura K, Kumai M, Morimoto S, Shibata Y. Conduction abnormality in gap junction protein connexin45-deficient embryonic stem cell-derived cardiac myocytes. Anat Rec A Discov Mol Cell Evol Biol. 2004; 280:973–9.

11). Coppen SR, Kodama I, Boyett MR, Dobrzynski H, Takagishi Y, Honjo H, et al. Connexin45, a major connexin of the rabbit sinoatrial node, is co-expressed with connexin43 in a restricted zone at the nodal-crista terminalis border. J Histochem Cytochem. 1999; 47:907–18.

12). Excoffon KJ, Hruska-Hageman A, Klotz M, Traver GL, Zabner J. A role for the PDZ-binding domain of the coxsackie B virus and adenovirus receptor (CAR) in cell adhesion and growth. J Cell Sci. 2004; 117:4401–9.

13). Lisewski U, Shi Y, Wrackmeyer U, Fischer R, Chen C, Schirdewan A, et al. The tight junction protein CAR regulates cardiac conduction and cell-cell communication. J Exp Med. 2008; 205:2369–79.

15). Knowlton KU, Jeon ES, Berkley N, Wessely R, Huber S. A mutation in the puff region of VP2 attenuates the myocarditic phenotype of an infectious cDNA of the Woodruff variant of coxsackievirus B3. J Virol. 1996; 70:7811–8.

16). Liu P, Martino T, Opavsky MA, Penninger J. Viral myocarditis: balance between viral infection and immune response. Can J Cardiol. 1996; 12:935–43.
17). Lim BK, Choe SC, Shin JO, Ho SH, Kim JM, Yu SS, et al. Local expression of interleukin-1 receptor antagonist by plasmid DNA improves mortality and decreases myocardial inflammation in experimental coxsackieviral myocarditis. Circulation. 2002; 105:1278–81.

18). He Y, Chipman PR, Howitt J, Bator CM, Whitt MA, Baker TS, et al. Interaction of coxsackievirus B3 with the full length coxsackievirus-adenovirus receptor. Nat Struct Biol. 2001; 8:874–8.
Go to : 
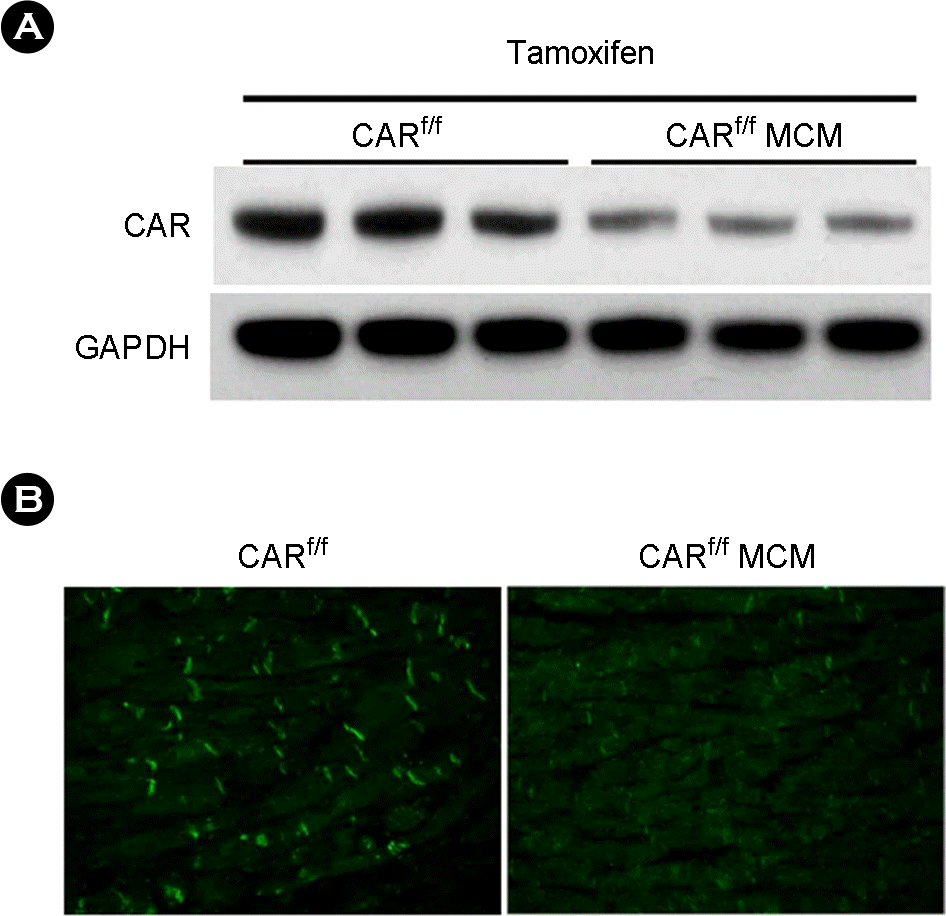 | Figure 1.Generation of Mice with Cardiac-Specific Deletion of CAR. After 2 weeks tamoxifen administration CAR expression was observed. CAR expression in CARf/f (control) and CARf/f MCM (Mutant) mice were measured by western blot analysis (A), and immunofluorescent staining (B) using an antibody specific for CAR (Green). The typical intercalated disc staining for CAR was observed in the CARf/f mice, but not in the CARf/f MCM mice. |
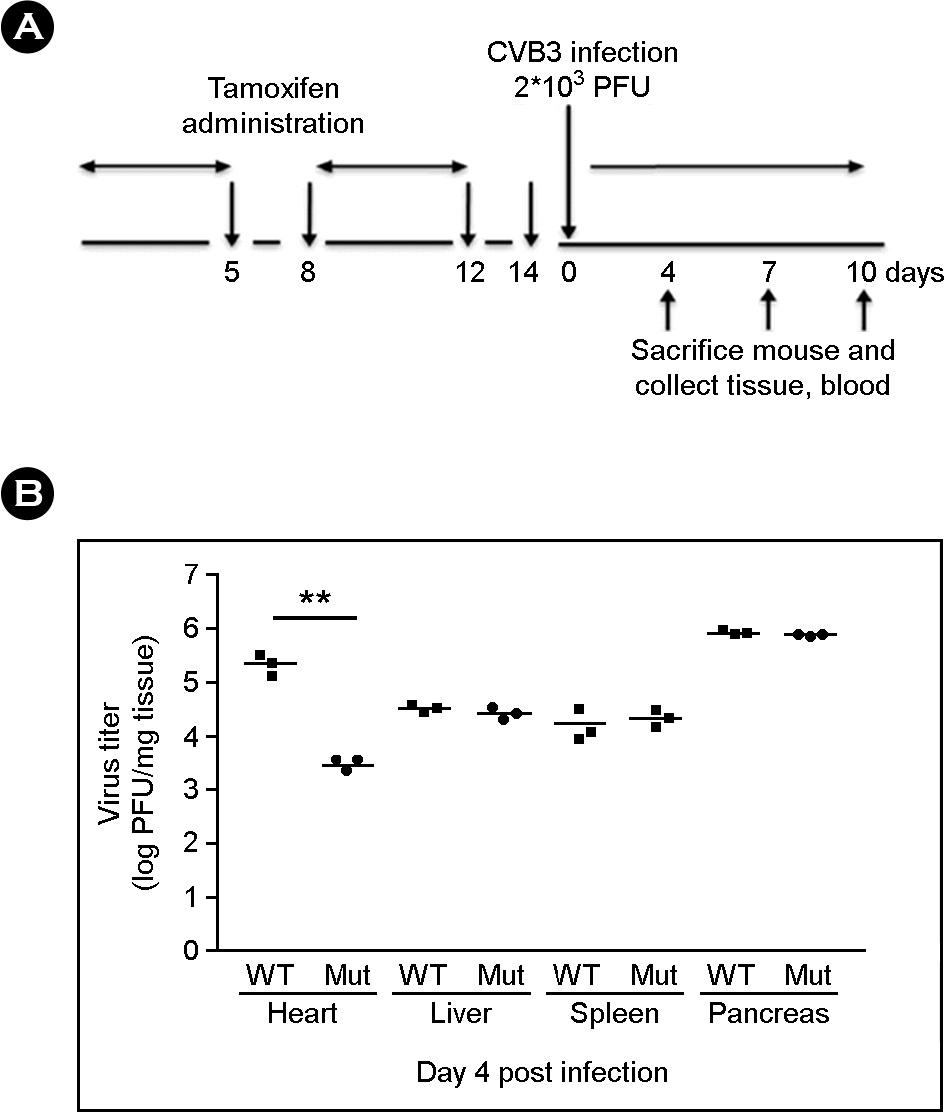 | Figure 2.Enterovirus infection in cardiac-specific CAR deletion mice. (A) Scheme of animal study for CAR deletion and enterovirus infection. Enterovirus was infected after 2 weeks tamoxifen administration. (B) Tissue virus titer was measured by PFU assay. In the CARf/f MCM mutant mice (Mut) heart virus titer was significantly reduced compare to CARf/f control mice (WT) heart 4 days following enterovirus infection. (Mean ± S.E.M, **p < 0.01, each data point is represented on graphs). |
 | Figure 3.Decrease cardiac myocyte damage. (A) Evans blue dye (EBD) uptake was significantly decreased in the heart of CARf/f MCM mutant mice 4 days following enterovirus infection compare to CARf/f control mice. (B) EBD uptake was measured in CARf/f mice (1.4 ± 0.5%) and CARf/f MCM mice (0.2 ± 0.1%). (Mean ± S.E.M, ***p < 0.001, each data point is represented on graphs). |
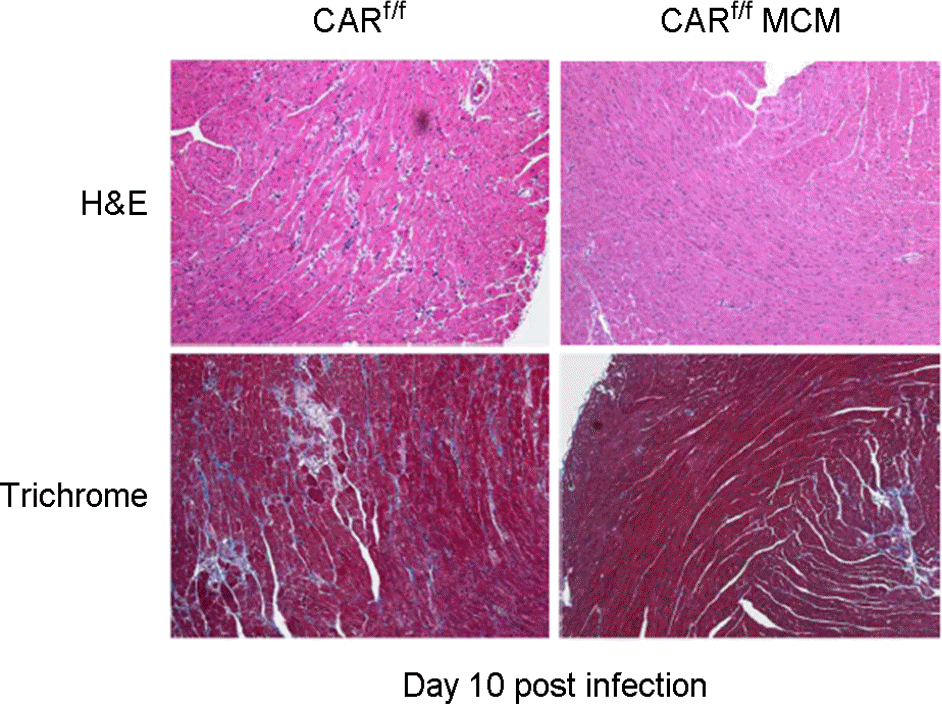 | Figure 4.Decrease inflammation and fibrosis. CAR deletion was effectively blocked enterovirus infection and proliferation in the heart. Inflammation and fibrosis were significantly reduced in CARf/f MCM mutant mice heart compare to CARf/f mice heart 10 days following enterovirus infection. Without CAR expression enterovirus was not able to infect into cardiac myocyte. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download