Abstract
Intracellular transduction of hydrophilic macromolecules has been problematic owing to the biochemical restriction imposed by lipid bilayer of the cytoplasmic membrane. Several technologies have been developed to improve the intracellular delivery of the large molecules for therapeutic purpose, including cell penetrating peptide. Cell penetrating peptides or cell permeable peptides (CPPs) were initially discovered based on the potency of certain full-length proteins or proteins to translocate across the plasma membrane. Currently, CPPs are broadly applied for intracellular delivery of biologically functional molecules in vivo and vitro, varying from small molecules, peptides, proteins, liposomes and nucleic acids. With introducing the history and characteristics of CPPs, this review will focus on the intracellular transduction mechanism and application of CPPs.
REFERENCES
1). Joliot A, Prochiantz A. Transduction peptides: from technology to physiology. Nat Cell Biol. 2004; 6:189–96.

3). Heitz F, Morris MC, Divita G. Twenty years of cell-penetrating peptides: from molecular mechanisms to therapeutics. Br J Pharmacol. 2009; 157:195–206.

4). Frankel AD, Pabo CO. Cellular uptake of the Tat protein from human immunodeficiency virus. Cell. 1988; 55:1189–93.

5). Futaki S, Suzuki T, Ohashi W, Yagami T, Tanaka S, Ueda K, et al. Arginine-rich peptides. An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J Biol Chem. 2001; 276:5836–40.
6). Berlose JP, Convert O, Derossi D, Brunissen A, Chassaing G. Conformational and associative behaviors of the third helix of Antennapedia homeodomain in membrane-mimetic environments. Eur J Biochem. 1996; 242:372–86.
7). Drin G, Déméné H, Temsamani J, Brasseur R. Translocation of the pAntp peptide and its amphipathic analogue AP-2AL. Biochemistry. 2001; 40:1824–34.

8). Scheller A, Wiesner B, Melzig M, Bienert M, Oehlke J. Evidence for an amphipathicity independent cellular uptake of amphipathic cell-penetrating peptides. Eur J Biochem. 2000; 267:6043–50.

9). Trabulo S, Cardoso AL, Mano M, de Lima MC. Cell-Penetrating Peptides-Mechanisms of Cellular Uptake and Generation of Delivery Systems. Pharmaceuticals. 2010; 3:961–93.

10). Vivès E, Brodin P, Lebleu B. A truncated HIV-1 Tat protein basic domain translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem. 1997; 272:16010–7.
11). Schmidt N, Mishra A, Lai GH, Wong GC. Arginine-rich cell-penetrating peptides. FEBS Lett. 2010; 584:1806–13.

12). Pietersz GA, Li W, Apostolopoulos V. A 16-mer peptide (RQIKIWFQNRRMKWKK) from antennapedia preferentially targets the Class I pathway. Vaccine. 2001; 19:1397–405.

13). Vasconcelos L, Pärn K, Langel U. Therapeutic potential of cell-penetrating peptides. Ther Deliv. 2013; 4:573–91.

14). Choi JM, Ahn MH, Chae WJ, Jung YG, Park JC, Song HM, et al. Intranasal delivery of the cytoplasmic domain of CTLA-4 using a novel protein transduction domain prevents allergic inflammation. Nat Med. 2006; 12:574–9.

16). Brugidou J. Legrand C, Méry J, Rabié A. The retro-inverso form of a homeobox-derived short peptide is rapidly internalised by cultured neurons: a new basis for an efficient intracellular delivery system. Biochem Biophys Res Commun. 1995; 214:685–93.
17). Suzuki T, Futaki S, Niwa M, Tanaka S, Ueda K, Sugiura Y. Possible existence of common internalization mechanisms among arginine-rich peptides. J Biol Chem. 2002; 277:2437–43.

18). Futaki S. Membrane-permeable arginine-rich peptides and the translocation mechanisms. Adv Drug Deliv Rev. 2005; 57:547–58.

19). Noguchi H, Matsushita M, Okitsu T, Moriwaki A, Tomizawa K, Kang S, et al. A new cell-permeable peptide allows successful allogeneic islet transplantation in mice. Nat Med. 2004; 10:305–9.

20). Jo D, Liu D, Yao S, Collins RD, Hawiger J. Intracellular protein therapy with SOCS3 inhibits inflammation and apoptosis. Nat Med. 2005; 11:892–8.

21). Järver P, Langel U. The use of cell-penetrating peptides as a tool for gene regulation. Drug Discov Today. 2004; 9:395–402.
22). Laufer SD, Restle T. Peptide-mediated cellular delivery of oligonucleotide-based therapeutics in vitro: quantitative evaluation of overall efficacy employing easy to handle reporter systems. Curr Pharm Des. 2008; 14:3637–55.
24). Juliano R, Alam MR, Dixit V, Kang H. Mechanisms and strategies for effective delivery of antisense and siRNA oligonucleotides. Nucleic Acids Res. 2008; 36:4158–71.

25). Kurreck J. Antisense technologies. Improvement through novel chemical modifications. Eur J Biochem. 2003; 270:1628–44.

26). Lebleu B, Moulton HM, Abes R, Ivanova GD, Abes S, Stein DA, et al. Cell penetrating peptide conjugates of steric block oligonucleotides. Adv Drug Deliv Rev. 2008; 60:517–29.

27). Torchilin VP. Cell penetrating peptide-modified pharmaceutical nanocarriers for intracellular drug and gene delivery. Biopolymers. 2008; 90:604–10.

28). Temsamani J, Vidal P. The use of cell-penetrating peptides for drug delivery. Drug Discov Today. 2004; 9:1012–9.

30). Dorsett Y, Tuschl T. siRNAs: applications in functional genomics and potential as therapeutics. Nat Rev Drug Discov. 2004; 3:318–29.

31). de Fougerolles A, Vornlocher HP, Maraganore J, Lieberman J. Interfering with disease: a progress report on siRNA-based therapeutics. Nat Rev Drug Discov. 2007; 6:443–53.

32). Muratovska A, Eccles MR. Conjugate for efficient delivery of short interfering RNA (siRNA) into mammalian cells. FEBS Lett. 2004; 558:63–8.

33). Chiu YL, Ali A, Chu CY, Cao H, Rana TM. Visualizing a correlation between siRNA localization, cellular uptake, and RNAi in living cells. Chem Biol. 2004; 11:1165–75.

34). Davidson TJ, Harel S, Arboleda VA, Prunell GF, Shelanski ML, Greene LA, et al. Highly efficient small interfering RNA delivery to primary mammalian neurons induces MicroRNA-like effects before mRNA degradation. J Neurosci. 2004; 24:10040–6.

35). Zeineddine D, Papadimou E, Chebli K, Gineste M, Liu J, Grey C, et al. Oct-3/4 dose dependently regulates specification of embryonic stem cells toward a cardiac lineage and early heart development. Dev Cell. 2006; 11:535–46.

Figure 1.
Utility of cell permeable peptide (CPP) in transduction of various cargos into the cell. In order to utilize the CPP in intracellular transduction of various cargo molecules, (A) CPP can be covalently linked to cargos by chemical synthesis. The disulfide bonds are also used for linkage between CPP and cargos. Especially, cargos of proteins are to be expressed with CPP as recombinant proteins. (B) A wide variety of cargo has been conjugated with arginine-dependent CPPs bearing lots of positive charges. Despite the mechanism of transduction across the cellular membrane is not clearly elucidated, the initial step (1) of intracellular transduction by CPP appears to involve charge-to-charge interaction between the basic amino-acids of CPP and acidic motifs on the cell membrane. The next step (2) remains to be studied, even though there have been several hypotheses with experimental evidences for explaining how the CPPs can be internalized into cell (refer to Fig. 2). Once inside the cell (3), numerous complex events and activities can be performed on the CPP and cargo such as target binding, separation of cargo from PTD, protein refolding, post-translational modification, macromolecular assembly, nuclear import/export and so on. (B) of this figure was modified from figure 1 of the reference (2).
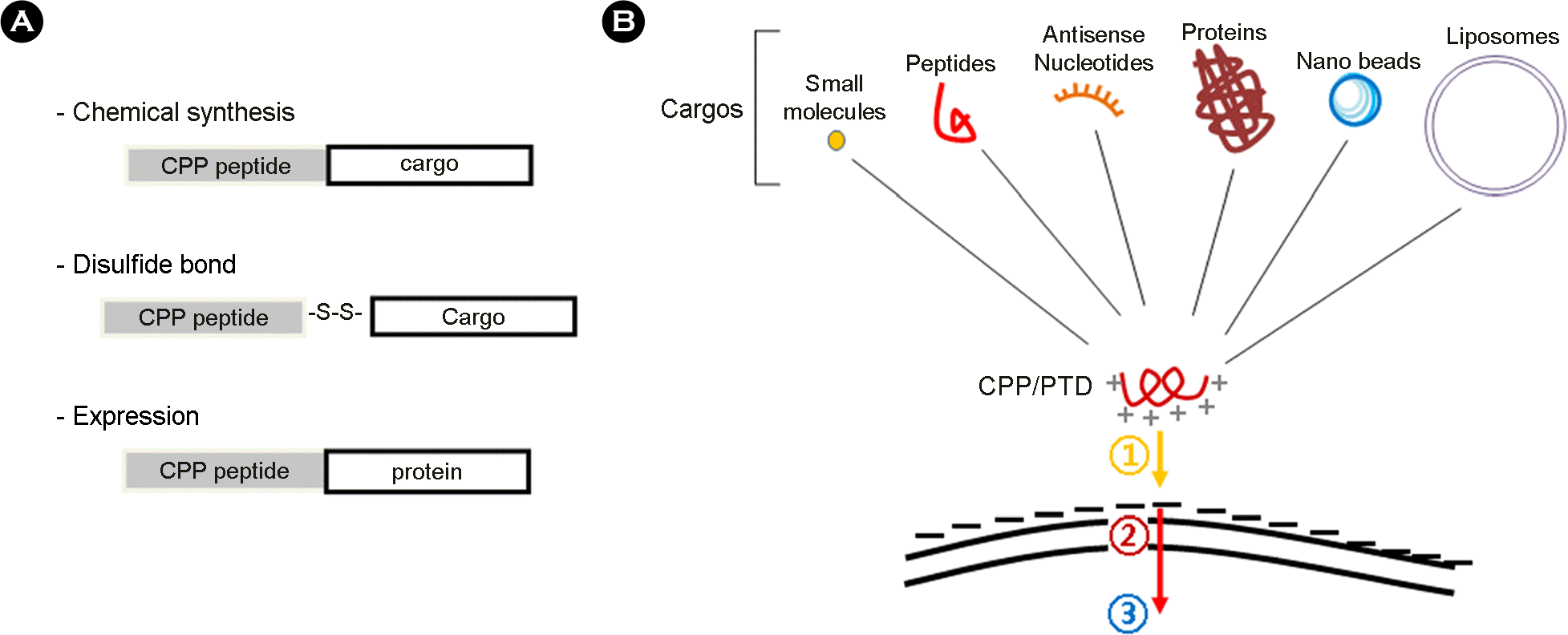
Figure 2.
Intracellular transduction mechanisms of cell permeable peptides. Intracellular transduction of CPPs has been explained with a variety of mechanisms. These mechanisms include (A) energy-dependent pathways, based on vesicle forming, known as endocytosis, and (B) direct translocation, which is engaged with the formation of hydrophilic pores or local destabilization of the cytoplasmic membrane. This figure was modified from figure 1 of the reference (9).
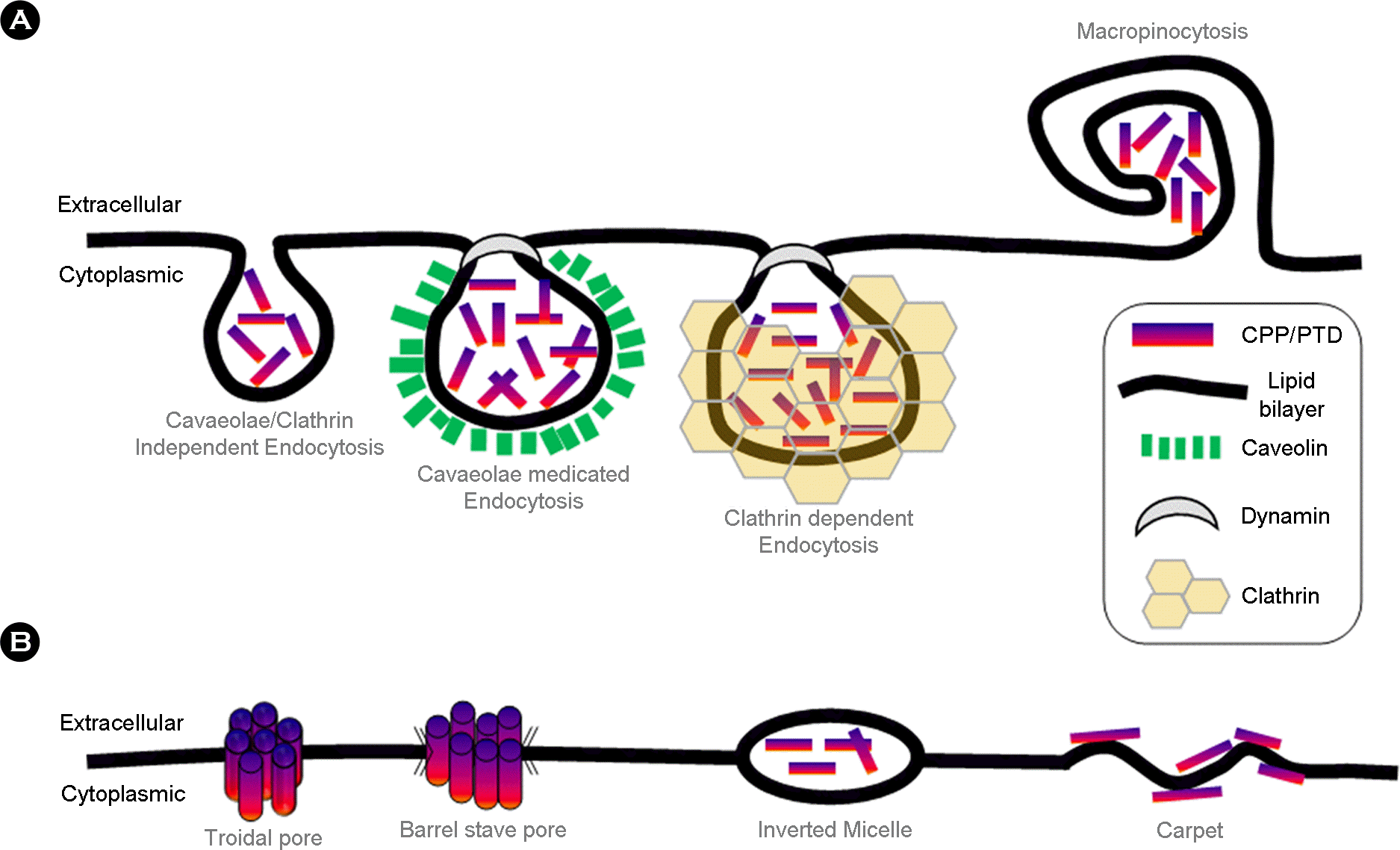
Table 1.
The representative cell-penetrating peptides (CPPs).
Table 2.
Therapeutic cases in animal models of diseases by utilizing cell-penetrating peptides (CPPs).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download