Abstract
Vibrio vulnificus is a Gram-negative halophilic bacterium that causes necrotizing wound infections and fatal septicemia, which mainly occur in patients with elevated serum or tissue iron levels. Accumulated experimental data clearly show that V. vulnificus is a ferrophilic bacterium that requires more available iron for growth than other pathogenic bacteria, has multiple iron-uptake systems, which play important roles in the pathogenesis of the V. vulnificus infections. This review summarized the composition, regulation and significance of V. vulnificus iron-uptake systems. These iron-uptake systems may be attractive candidates for the development of V. vulnificus vaccine. Iron-chelating therapy can also be a promising modality for the prevention and treatment of V. vulnificus infections.
REFERENCES
2). Bagg A, Neilands JB. Molecular mechanism of regulation of siderophore-mediated iron assimilation. Microbiol Rev. 1987; 51:509–18.

4). Gray-Owen SD, Schryvers AB. Bacterial transferrin and lactoferrin receptors. Trends Microbiol. 1996; 4:185–91.

5). Mey AR, Payne SM. Haem utilization in Vibrio cholerae involves multiple TonB-dependent haem receptors. Mol Microbiol. 2001; 42:835–49.
6). Litwin CM, Byrne BL. Cloning and characterization of an outer membrane protein of Vibrio vulnificus required for heme utilization: regulation of expression and determination of the gene sequence. Infect Immun. 1998; 66:3134–41.
7). Perkins-Balding D, Baer MT, Stojiljkovic I. Identification of functionally important regions of a haemoglobin receptor from Neisseria meningitidis. Microbiology. 2003; 149:3423–35.
11). Wooldridge KG, Williams PH. Iron uptake mechanisms of pathogenic bacteria. FEMS Microbiol Rev. 1993; 12:325–48.

12). Schubert S, Fischer D, Heesemann J. Ferric enterohelin transport in Yersinia enterocolitica: Molecular and evolutionary aspects. J Bacteriol. 1999; 181:6387–95.
13). Aso H, Miyoshi S, Nakao H, Okamoto K, Yamamoto S. Induction of an outer membrane protein of 78 kDa in Vibrio vulnificus cultured in the presence of desferrioxamine B under iron-limiting conditions. FEMS Microbiol Lett. 2002; 212:65–70.
14). Tanabe T, Naka A, Aso H, Nakao H, Narimatsu S, Inoue Y, et al. A novel aerobactin utilization cluster in Vibrio vulnificus with a gene involved in the transcription regulation of the iutA homologue. Microbiol Immunol. 2005; 49:823–34.
15). Tanabe T, Takata N, Naka A, Moon YH, Nakao H, Inoue Y, et al. Identification of an AraC-like regulator gene required for induction of the 78-kDa ferrioxamine B receptor in Vibrio vulnificus. FEMS Microbiol Lett. 2005; 249:309–14.
16). Kim CM, Shin SH. Effect of iron-chelator deferiprone on the in vitro growth of staphylococci. J Korean Med Sci. 2009; 24:289–95.
17). Kim CM, Park YJ, Shin SH. A widespread deferoxamine-mediated iron-uptake system in Vibrio vulnificus. J Infect Dis. 2007; 196:1537–45.
18). Braun V, Hantke K, Köster W. Bacterial iron transport: mechanisms, genetics, and regulation. Met Ions Biol Syst. 1998; 35:67–145.
19). Andrews SC, Robinson AK, Rodríguez-Quiñones F. Bacterial iron homeostasis. FEMS Microbiol Rev. 2003; 27:215–37.

20). Choi MH, Sun HY, Park RY, Kim CM, Bai YH, Kim YR, et al. Effect of the crp mutation on the utilization of transferrin-bound iron by Vibrio vulnificus. FEMS Microbiol Lett. 2006; 257:285–92.
21). Oh MH, Lee SM, Lee DH, Choi SH. Regulation of the Vibrio vulnificus hupA gene by temperature alteration and cyclic AMP receptor protein and evaluation of its role in virulence. Infect Immun. 2009; 77:1208–15.
22). Kim CM, Kim SJ, Shin SH. Cyclic AMP-receptor protein activates aerobactin receptor IutA expression in Vibrio vulnificus. J Microbiol. 2012; 50:320–5.
23). Kim SP, Lee GW, Kim CM, Shin SH. Coordinate regulation of Vibrio vulnificus heme receptor HupA expression by cyclic AMP-receptor protein and ferric uptake regulator. J Bacteriol Virol. 2012; 42:294–304.
24). Kim CM, Shin SH. Modulation of iron-uptake systems by a mutation of luxS encoding an autoinducer-2 synthase in Vibrio vulnificus. Biol Pharm Bull. 2011; 34:632–7.
25). Wen Y, Kim IH, Son JS, Lee BH, Kim KS. Iron and quorum sensing coordinately regulate the expression of vulnibactin biosynthesis in Vibrio vulnificus. J Biol Chem. 2012; 287:26727–39.
26). Hlady WG, Klontz KC. The epidemiology of Vibrio infections in Florida, 1981–1993. J Infect Dis. 1996; 173:1176–83.
27). Strom MS, Paranjpye RN. Epidemiology and pathogenesis of Vibrio vulnificus. Microbes Infect. 2000; 2:177–88.
28). Gulig PA, Bourdage KL, Starks AM. Molecular pathogenesis of Vibrio vulnificus. J Microbiol. 2005; 43:118–31.
29). Brennt CE, Wright AC, Dutta SK, Morris JG Jr. Growth of Vibrio vulnificus in serum from alcoholics: association with high transferrin iron saturation. J Infect Dis. 1991; 164:1030–2.
30). Hor LI, Chang TT, Wang ST. Survival of Vibrio vulnificus in whole blood from patients with chronic liver diseases: association with phagocytosis by neutrophils and serum ferritin levels. J Infect Dis. 1999; 179:275–8.
31). Hor LI, Chang YK, Chang CC, Lei HY, Ou JT. Mechanism of high susceptibility of iron-overloaded mouse to Vibrio vulnificus infection. Microbiol Immunol. 2000; 44:871–8.
32). Wright AC, Simpson LM, Oliver JD. Role of iron in the pathogenesis of Vibrio vulnificus infections. Infect Immun. 1981; 34:503–7.
33). Starks AM, Schoeb TR, Tamplin ML, Parveen S, Doyle TJ, Bomeisl PE, et al. Pathogenesis of infection by clinical and environmental strains of Vibrio vulnificus in iron-dextran-treated mice. Infect Immun. 2000; 68:5785–93.
34). Kim CM, Chung YY, Shin SH. Iron differentially regulates gene expression and extracellular secretion of Vibrio vulnificus cytolysin-hemolysin. J Infect Dis. 2009; 200:582–9.
35). Kim CM, Kim SC, Shin SH. Iron-mediated regulation of metalloprotease VvpE production in Vibrio vulnificus. New Microbiol. 2012; 35:481–6.
36). Kim CM, Park RY, Choi MH, Sun HY, Shin SH. Ferrophilic characteristics of Vibrio vulnificus and potential usefulness of iron chelation therapy. J Infect Dis. 2007; 195:90–8.
37). Weinberg ED. Microbial pathogens with impaired ability to acquire host iron. BioMetals. 2000; 13:85–9.
38). Simpson LM, Oliver JD. Siderophore production by Vibrio vulnificus. Infect Immun. 1983; 41:644–9.
39). Okujo N, Saito M, Yamamoto S, Yoshida T, Miyoshi S, Shinoda S. Structure of vulnibactin, a new polyamine-containing siderophore from Vibrio vulnificus. Biometals. 1994; 7:109–16.

40). Stelma GN Jr, Reyes AL, Peeler JT, Johnson CH, Spaulding PL. Virulence characteristics of clinical and environmental isolates of Vibrio vulnificus. Appl Environ Microbiol. 1992; 58:2776–82.
41). Shin SH, Chung SS, Rhee JH. Phenolate siderophore stimulates growth of Vibrio vulnificus: Application of CAS agar diffusion assay-Comparison of siderophore production among strains. J Bacteriol Virol. 2001; 4:325–31.
42). Litwin CM, Rayback TW, Skinner J. Role of catechol siderophore synthesis in Vibrio vulnificus virulence. Infect Immun. 1996; 64:2834–8.
43). Sun HY, Han SI, Choi MH, Kim SJ, Kim CM, Shin SH. Vibrio vulnificus metalloprotease VvpE has no direct effect on iron-uptake from human hemoglobin. J Microbiol. 2006; 44:537–47.
44). Kim CM, Park RY, Park JH, Sun HY, Bai YH, Ryu PY, et al. Vibrio vulnificus vulnibactin, but not metalloprotease VvpE, is essentially required for iron-uptake from human holotransferrin. Biol Pharm Bull. 2006; 29:911–8.
45). Litwin CM, Calderwood SB. Role of iron in regulation of virulence genes. Clin Microbiol Rev. 1993; 6:137–49.

46). Webster AC, Litwin CM. Cloning and characterization of vuuA, a gene encoding the Vibrio vulnificus ferric vulnibactin receptor. Infect Immun. 2000; 68:526–34.
47). Lee HJ, Lee KH. Identification of the fur-binding site in regulatory region of the vulnibactin-receptor gene in Vibrio vulnificus. J Microbiol Biotechnol. 2012; 22:46–9.
48). Lesic B, Foulon J, Carniel E. Comparison of the effects of deferiprone versus deferoxamine on growth and virulence of Yersinia enterocolitica. Antimicrob Agents Chemother. 2002; 46:1741–5.
49). Kim DM, Cho HS, Kang JI, Kim HS, Park CY. Deferasirox plus ciprofloxacin combination therapy after rapid diagnosis of Vibrio vulnificus sepsis using real-time polymerase chain reaction. J Infect. 2008; 57:489–92.
50). Neupane GP, Kim DM. Comparison of the effects of deferasirox, deferiprone, and deferoxamine on the growth and virulence of Vibrio vulnificus. Transfusion. 2009; 49:1762–9.
51). Neupane GP, Kim DM. In vitro time-kill activities of ciprofloxacin alone and in combination with the iron chelator deferasirox against Vibrio vulnificus. Eur J Clin Microbiol Infect Dis. 2010; 29:407–10.
52). Litwin CM, Quackenbush J. Characterization of a Vibrio vulnificus LysR homologue, HupR, which regulates expression of the haem uptake outer membrane protein, HupA. Microb Pathog. 2001; 31:295–307.
53). Datta S, Crosa JH. Identification and characterization of a novel outer membrane protein receptor required for hemin utilization in Vibrio vulnificus. Biometals. 2012; 25:275–83.
54). Martínez JL, Delgado-Iribarren A, Baquero F. Mechanisms of iron acquisition and bacterial virulence. FEMS Microbiol Rev. 1990; 6:45–56.

55). Wright AC, Morris JG Jr. The extracellular cytolysin of Vibrio vulnificus: inactivation and relationship to virulence in mice. Infect Immun. 1991; 59:192–7.
56). Lee SE, Ryu PY, Kim SY, Kim YR, Koh JT, Kim OJ, et al. Production of Vibrio vulnificus hemolysin in vivo and its pathogenic significance. Biochem Biophys Res Commun. 2004; 324:86–91.
57). Choi MH, Park RY, Sun HY, Kim CM, Bai YH, Lee SE, et al. Suppression and inactivation of Vibrio vulnificus hemolysin in cirrhotic ascites, a human ex vivo experimental system. FEMS Immunol Med Microbiol. 2006; 47:226–32.
58). Yamamoto K, Wright AC, Kaper JB, Morris JG Jr. The cytolysin gene of Vibrio vulnificus: sequence and relationship to the Vibrio cholerae E1 Tor hemolysin gene. Infect Immun. 1990; 58:2706–9.
59). Lee HJ, Kim JA, Lee MA, Park SJ, Lee KH. Regulation of haemolysin (VvhA) production by ferric uptake regulator (Fur) in Vibrio vulnificus: repression of vvhA transcription by Fur and proteolysis of VvhA by Fur-repressive exoproteases. Mol Microbiol. 2013; 88:813–26.
60). Okujo N, Akiyama T, Miyoshi S, Shinoda S, Yamamoto S. Involvement of vulnibactin and exocellular protease in utilization of transferrin- and lactoferrin-bound iron by Vibrio vulnificus. Microbiol Immunol. 1996; 40:595–8.
61). Shin SH, Sun HY, Park RY, Kim CM, Kim SY, Rhee JH. Vibrio vulnificus metalloprotease VvpE has no direct effect on the iron-assimilation from human holotransferrin. FEMS Microbiol Lett. 2005; 247:221–9.
62). Kim IH, Wen Y, Son JS, Lee KH, Kim KS. The Fur-iron complex modulates expression of the quorum-sensing master regulator, SmcR, to control expression of virulence factors in Vibrio vulnificus. Infect Immun. 2013; 81:2888–98.
63). Rokbi B, Mignon M, Maitre-Wilmotte G, Lissolo L, Danve B, Caugant DA, et al. Evaluation of recombinant transferrin-binding protein B variants from Neisseria meningitidis for their ability to induce cross-reactive and bactericidal antibodies against a genetically diverse collection of serogroup B strains. Infect Immun. 1997; 65:55–63.
64). Myers LE, Yang YP, Du RP, Wang Q, Harkness RE, Schryvers AB, et al. The transferrin binding protein B of Moraxella catarrhalis elicits bactericidal antibodies and is a potential vaccine antigen. Infect Immun. 1998; 66:4183–92.
65). Brown JS, Ogunniyi AD, Woodrow MC, Holden DW, Paton JC. Immunization with components of two iron uptake ABC transporters protects mice against systemic Streptococcus pneumoniae infection. Infect Immun. 2001; 69:6702–6.
66). Kuboniwa M, Amano A, Shizukuishi S, Nakagawa I, Hamada S. Specific antibodies to Porphyromonas gingivalis Lysgingipain by DNA vaccination inhibit bacterial binding to hemoglobin and protect mice from infection. Infect Immun. 2001; 69:2972–9.
Figure 1.
The five iron-uptake systems (IUS) of Vibrio vulnificus. Vulnibactin (VuuA)-mediated IUS (purple), hydroxamate siderophore-mediated IUS (white; the question mark means “unknown yet.”), heme receptor (HupA)-mediated IUS (deep blue), aerobactin receptor (IutA)-mediated IUS (sky blue), and Ferrioxamine B receptor (DesA)-mediated IUS (green).
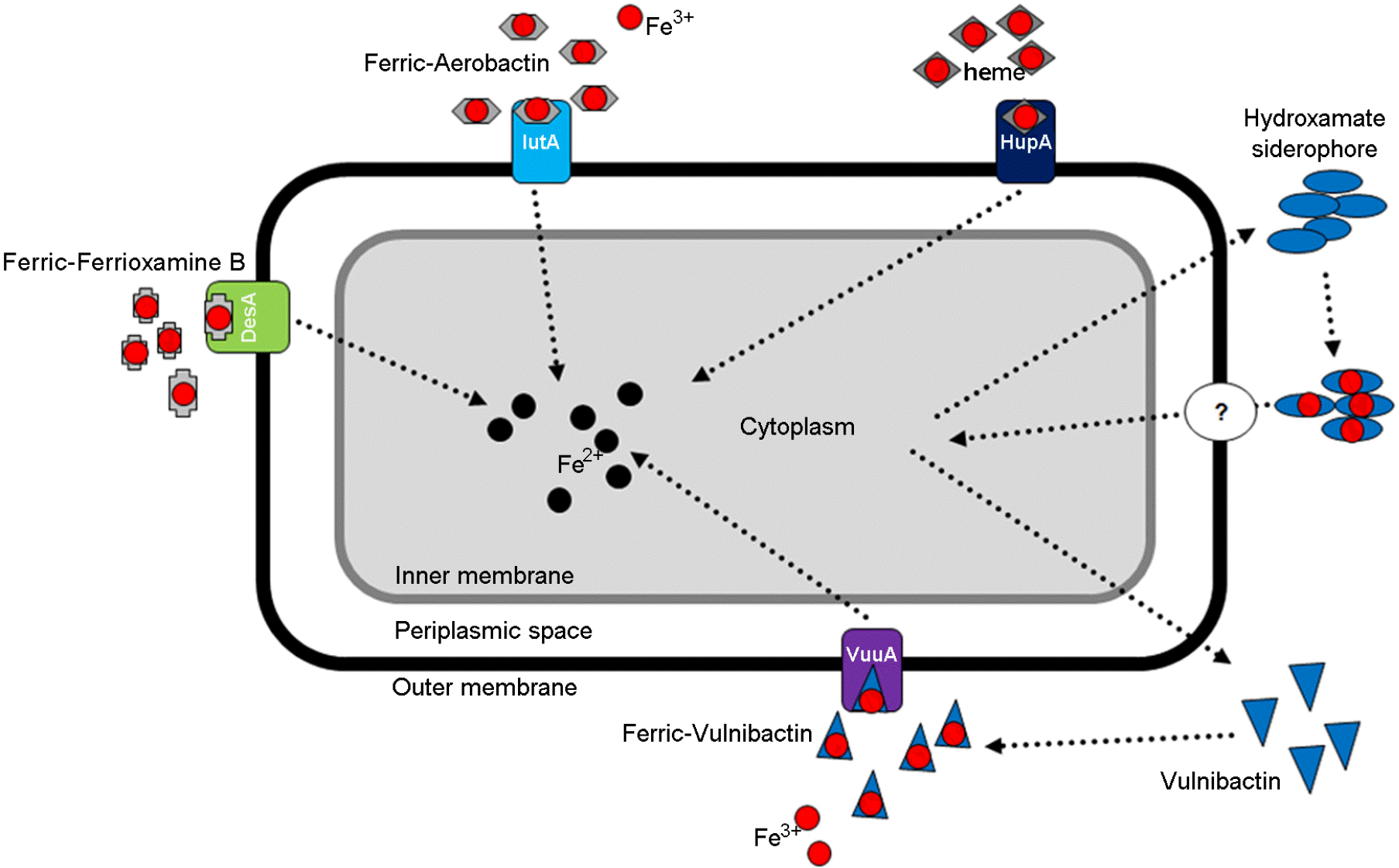
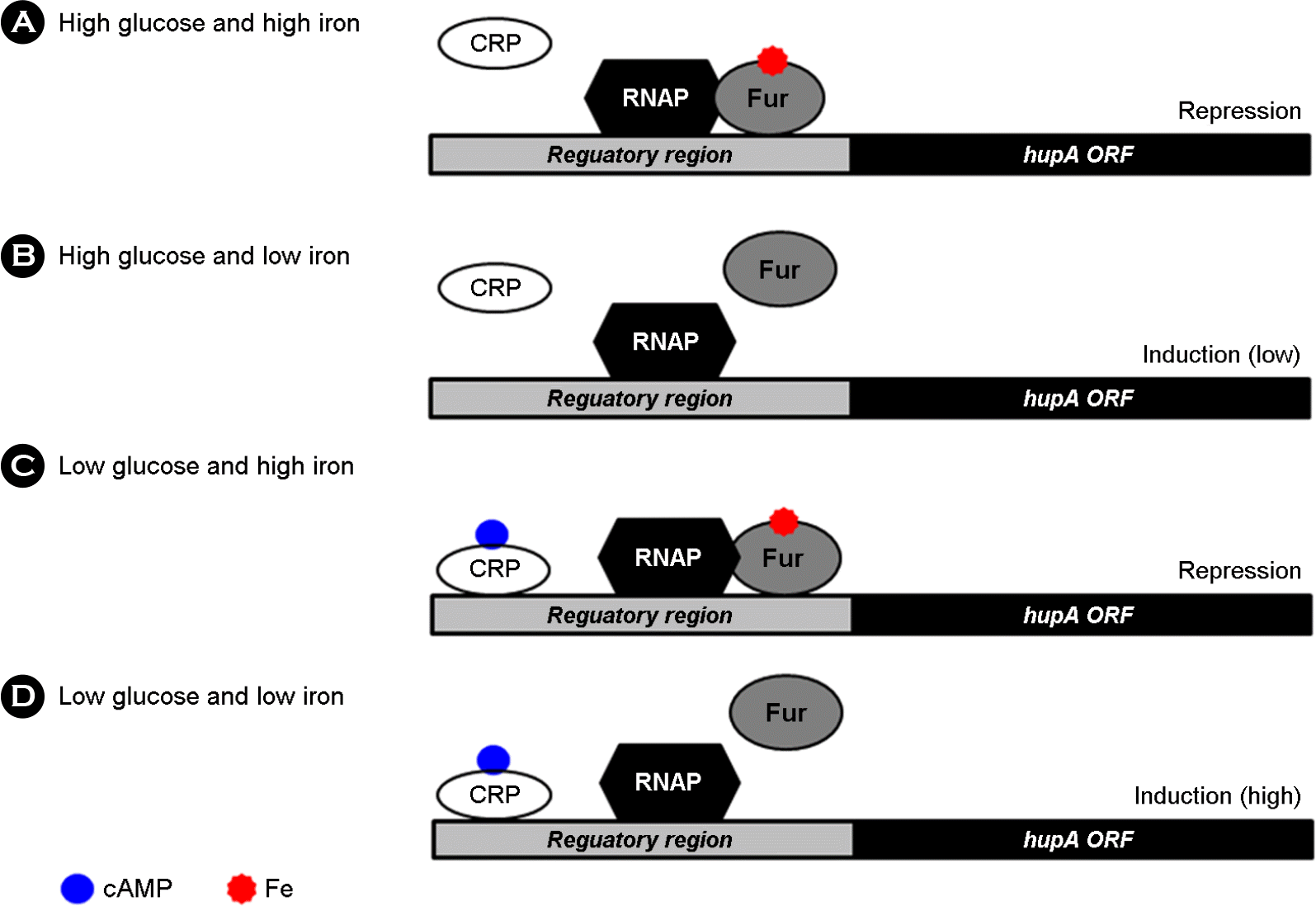
Figure 2.
Coordinate regulation of hupA expression by cyclic AMP-receptor protein (CRP) as an activator and ferric uptake regulator (Fur) as a repressor. (A) High glucose prevents the binding of CRP and high iron facilitates the binding of Fur, resulting in the repression of hupA expression. (B) High glucose prevents the binding of CRP but low iron prevents the binding of Fur, resulting in the low induction (or de-repression) of hupA expression. (C) Low glucose facilitates the binding of CRP but high iron facilitates the binding of Fur, resulting in the repression of hupA expression. (D) Low glucose facilitates the binding of CRP and low iron prevents the binding of Fur, resulting in the high induction (or de-repression) of hupA expression. RNAP (RNA polymerase) and ORF (open reading frame). Quoted from the reference 23.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download