Abstract
Some Bacillus species present in fermented foods are regarded as probiotics because of their ability to modulate the prevention of some intestinal infections and the modulation of the inflammatory immune response. We isolated bacteriocin-like substances producing Bacillus subtilis and B. lentus from Cheonggukjang, a traditional Korean fermented soybean paste having an inhibitory effect against Salmonella Typhimurium using a well diffusion inhibition assay and a broth co-culturing method. B. subtilis or B. letus was fed to Drosophila melanogaster alone as well as in combination with Salmonella Typhimurium and survival was monitored daily. The survival rates by oral feeding B. subtilis, B. lentus and Salmonella Typhimurium separately resulted in 85, 90 and 75%, respectively. In contrast, survival rates of co-feeding of B. lentus with Salmonella Typhimurium were increased from 75 to 90% during 7 days post-feeding as compared to Salmonella Typhimurium alone. However, B. subtilis in co-feeding with Salmonella Typhimurium significantly reduced D. melanogaster survival rate (85 to 70%). We found that the immune response to B. lentus and Salmonella Typhimurium is characterized synergistic activation of antimicrobial peptide gene expression by Imd pathway. In conclusion, the in vitro and natural-route infection of the D. melanogaster digestive system can result in the use of the probiotic B. lentus for effective treatment of Salmonella Typhimurium infection. We therefore propose the strain B. lentus as a suitable candidate probiotics for use in the prevention and treatment of the intestinal infections caused by Salmonella Typhimurium.
REFERENCES
1). Kim S. Salmonella serovars from foodborne and waterborne diseases in Korea, 1998–2007: total isolates decreasing versus rare serovars emerging. J Korean Med Sci. 2010; 25:1693–9.
2). Atassi F, Servin AL. Individual and co-operative roles of lactic acid and hydrogen peroxide in the killing activity of enteric strain Lactobacillus johnsonii NCC933 and vaginal strain Lactobacillus gasseri KS120.1 against enteric, uropathogenic and vaginosis-associated pathogens. FEMS Microbiol Lett. 2010; 304:29–38.
3). Chenoll E, Casinos B, Bataller E, Astals P, Echevarría J, Iglesias JR, et al. Novel probiotic Bifidobacterium bifidum CECT 7366 strain active against the pathogenic bacterium Helicobacter pylori. Appl Environ Microbiol. 2011; 77:1335–43.
4). Pineiro M, Stanton C. Probiotic bacteria: legislative framework– requirements to evidence basis. J Nutr. 2007; 137:850S–3S.

5). Jung JY, Lee SH, Kim JM, Park MS, Bae JW, Hahn Y, et al. Metagenomic analysis of kimchi, a traditional Korean fermented food. Appl Environ Microbiol. 2011; 77:2264–74.

6). Song YR, Song NE, Kim JH, Nho YC, Baik SH. Exopolysaccharide produced by Bacillus licheniformis strains isolated from Kimchi. J Gen Appl Microbiol. 2011; 57:169–75.
7). Kim TW, Lee JH, Park MH, Kim HY. Analysis of bacterial and fungal communities in Japanese- and Chinese-fermented soybean pastes using nested PCR-DGGE. Curr Microbiol. 2010; 60:315–20.

8). Kwon GH, Lee HA, Park JY, Kim JS, Lim J, Park CS, et al. Development of a RAPD-PCR method for identification of Bacillus species isolated from Cheonggukjang. Int J Food Microbiol. 2009; 129:282–7.

10). Reva ON, Smirnov VV, Pettersson B, Priest FG. Bacillus endophyticus sp. nov., isolated from the inner tissues of cotton plants (Gossypium sp.). Int J Syst Evol Microbiol. 2002; 52:101–7.
11). Hong HA, To E, Fakhry S, Baccigalupi L, Ricca E, Cutting SM. Defining the natural habitat of Bacillus spore-formers. Res Microbiol. 2009; 160:375–9.

12). Jones SE, Knight KL. Bacillus subtilis-mediated protection from Citrobacter rodentium-associated enteric disease requires espH and functional flagella. Infect Immun. 2012; 80:710–9.
13). Deng W, Dong XF, Tong JM, Zhang Q. The probiotic Bacillus licheniformis ameliorates heat stress-induced impairment of egg production, gut morphology, and intestinal mucosal immunity in laying hens. Poult Sci. 2012; 91:575–82.
14). Wolfenden RE, Pumford NR, Morgan MJ, Shivaramaiah S, Wolfenden AD, Pixley CM, et al. Evaluation of selected direct-fed microbial candidates on live performance and Salmonella reduction in commercial turkey brooding houses. Poult Sci. 2011; 90:2627–31.

15). Nithya V, Muthukumar SP, Halami PM. Safety assessment of Bacillus licheniformis Me1 isolated from milk for probiotic application. Int J Toxicol. 2012; 31:228–37.
16). Sorokulova IB, Pinchuk IV, Denayrolles M, Osipova IG, Huang JM, Cutting SM, et al. The safety of two Bacillus probiotic strains for human use. Dig Dis Sci. 2008; 53:954–63.

17). Nagpal R, Kumar A, Kumar M, Behare PV, Jain S, Yadav H. Probiotics, their health benefits and applications for developing healthier foods: a review. FEMS Microbiol Lett. 2012; 334:1–15.

18). Apidianakis Y, Rahme LG. Drosophila melanogaster as a model for human intestinal infection and pathology. Dis Model Mech. 2011; 4:21–30.
19). Charroux B, Royet J. Gut-microbiota interactions in nonmammals: what can we learn from Drosophila? Semin Immunol. 2012; 24:17–24.

20). Choi YH, Chung J, Na HS. Molecular methods for studying the human microbiota. J Bacteriol Virol. 2013; 43:67–72.

21). Lavermicocca P, Valerio F, Lonigro SL, Di Leo A, Visconti A. Antagonistic activity of potential probiotic Lactobacilli against the ureolytic pathogen Yersinia enterocolitica. Curr Microbiol. 2008; 56:175–81.
22). Abriouel H, Franz CM, Ben Omar N, Gálvez A. Diversity and applications of Bacillus bacteriocins. FEMS Microbiol Rev. 2011; 35:201–32.
23). Kamada N, Kim YG, Sham HP, Vallance BA, Puente JL, Martens EC, et al. Regulated virulence controls the ability of a pathogen to compete with the gut microbiota. Science. 2012; 336:1325–9.

24). Bernet-Camard MF, Liévin V, Brassart D, Neeser JR, Servin AL, Hudault S. The human Lactobacillus acidophilus strain LA1 secretes a nonbacteriocin antibacterial substance(s) active in vitro and in vivo. Appl Environ Microbiol. 1997; 63:2747–53.
25). van de Guchte M, Ehrlich SD, Maguin E. Production of growth-inhibiting factors by Lactobacillus delbrueckii. J Appl Microbiol. 2001; 91:147–53.
27). Kreuzer S, Machnowska P, Aßmus J, Sieber M, Pieper R, Schmidt MF, et al. Feeding of the probiotic bacterium Enterococcus faecium NCIMB 10415 differentially affects shedding of enteric viruses in pigs. Vet Res. 2012; 43:58.
28). Apidianakis Y, Rahme LG. Drosophila melanogaster as a model for human intestinal infection and pathology. Dis Model Mech. 2011; 4:21–30.
29). Frandsen JL, Gunn B, Muratoglu S, Fossett N, Newfeld SJ. Salmonella pathogenesis reveals that BMP signaling regulates blood cell homeostasis and immune responses in Drosophila. Proc Natl Acad Sci U S A. 2008; 105:14952–7.

30). Liehl P, Blight M, Vodovar N, Boccard F, Lemaitre B. Prevalence of local immune response against oral infection in a Drosophila/Pseudomonas infection model. PLoS Pathog. 2006; 2:e56.

31). Nehme NT, Liégeois S, Kele B, Giammarinaro P, Pradel E, Hoffmann JA, et al. A model of bacterial intestinal infections in Drosophila melanogaster. PLoS Pathog. 2007; 3:e173.
Figure 1.
Detection of antimicrobial activity of B. subtilis (BS) or B. lentus (BL) with Salmonella Typhimurium (ST) by agar well diffusion assay. The indicator organism (Salmonella Typhimurium) in the 0.7% soft agar was spread on the surface of solid agar medium. Pasteur pipette was used to create 5 mm wells in the overlaid base agar plate. The well was filled with 30 μl of the cell free supernatant of Bacillus species. The plates were incubated and examined for the clear zones around the wells. Bacillus species isolated from a traditional fermented soybean paste displayed a wide range of antimicrobial activity against Salmonella Typhimurium. S. typhi: Salmonella typhimurium, 1800: Bacillus subtilis, 1900: Bacillus lentus-1, 2720: Bacillus lentus-2.
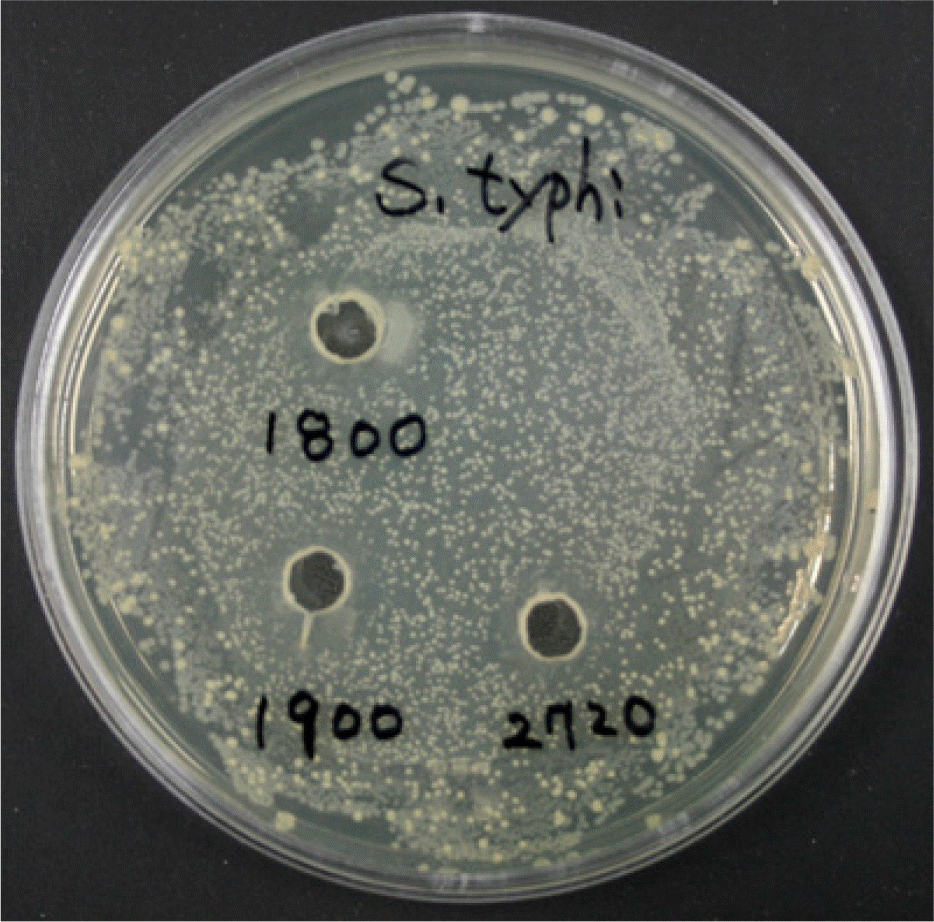
Figure 2.
The effect of co-cultured B. subtilis (BS) or B. lentus (BL) with Salmonella Typhimurium (ST) to inhibit the growth of ST in broth. BS or BL were mixed at a ratio of approximately 1:1 and co-cultured in broth at 24 h interval for 7 days. They were plated on MacConkey agar plate to determine viable cell count of ST. BL resulted in the complete killing of ST within 7 days co-cultured in broth, but BS did not observed the bactericidal effect.
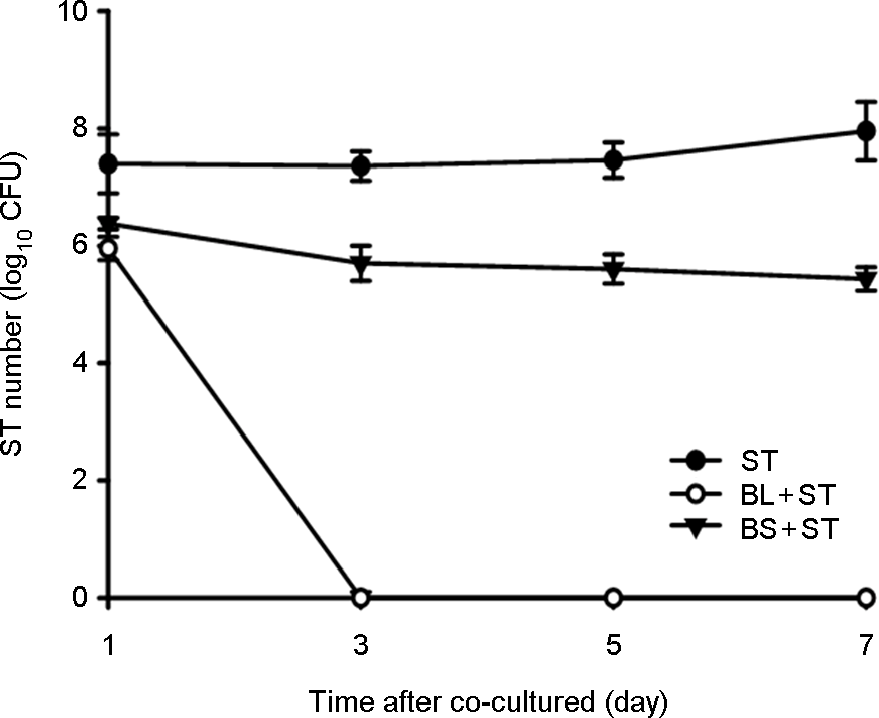
Figure 3.
Survival analysis of D. melanogaster that were oral feeding with B. subtilis (BS), B. lentus (BL), Salmonella Typhimurium (ST), BS/ST and BL/ST. Adult female flies were fed 5% sucrose in nutrient broth containing BS, BL, ST, BS/ST and BL/ST for 24 h, then transferred to sterile food. Survival rate (%) were determined over indicated time.
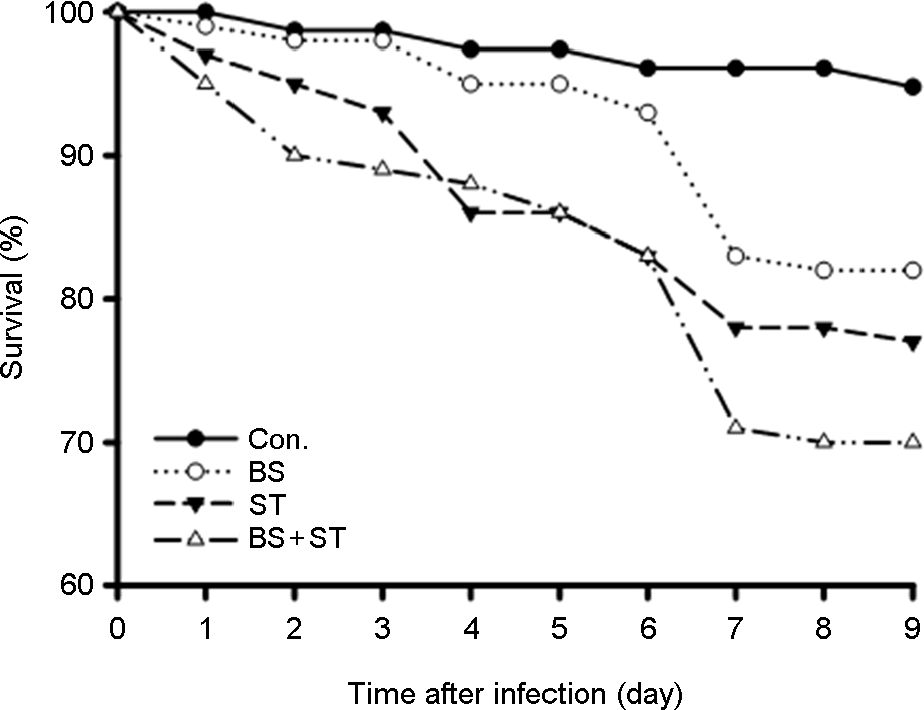
Figure 4.
Effect of oral feeding with B. lentus (BL) or B. subtilis (BS) on eradication of Salmonella Typhimurium (ST) in D. melanogaster. Adult female flies were fed 5% sucrose in nutrient broth containing ST, BL/ST and BS/ST for 24 h, and then transferred to sterile food. Guts were dissected and crushed at various times after ingestion using micropestle, and then homogenate was serially diluted in LB medium. The number of colony forming units (CFU) was determined through growth overnight at 37°C on MacConkey agar.
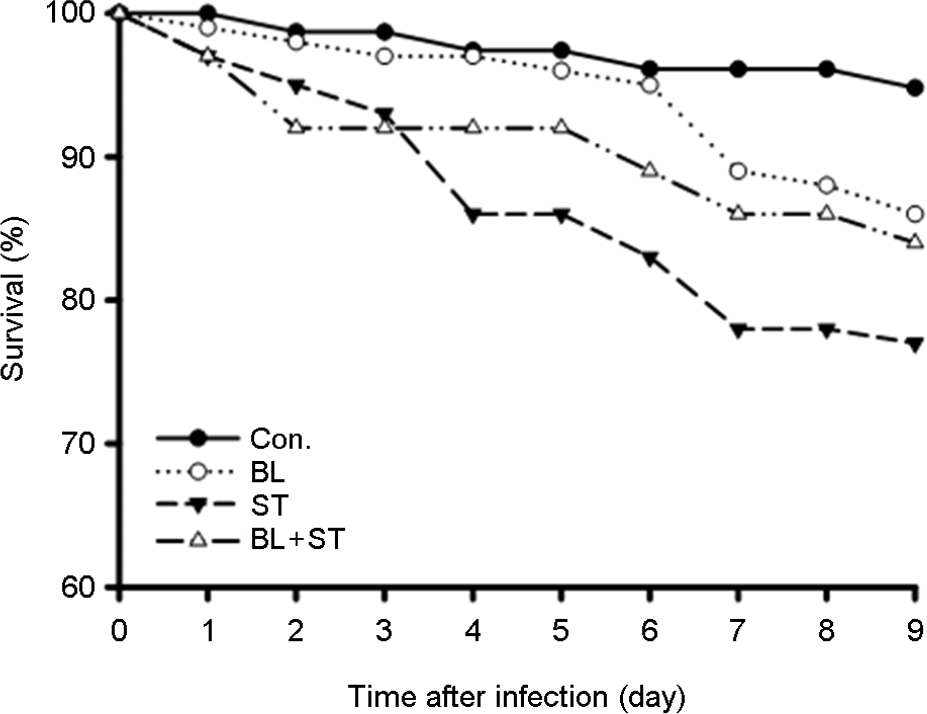
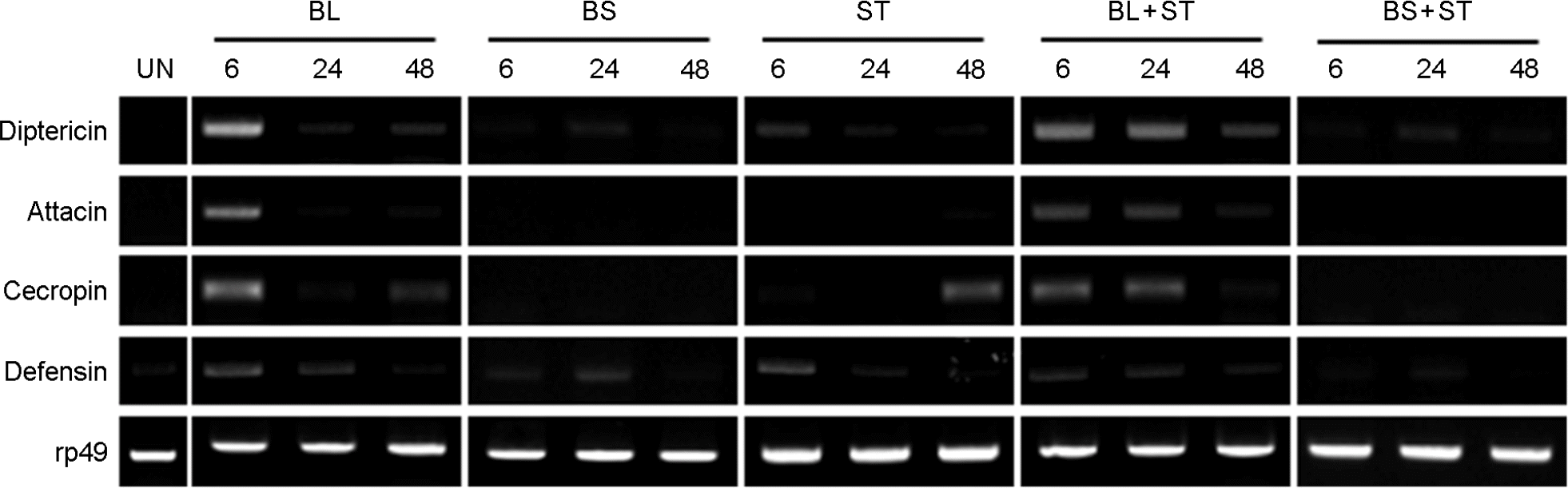
Figure 5.
Synergistic induction of antimicrobial peptides (AMPs) genes of D. melanogaster that were oral feeding with B. subtilis (BS), B. lentus (BL), Salmonella Typhimurium (ST), BS/ST and BL/ST. Total RNA was isolated from fly intestine treated with different combinations of BS, BL and ST for 6, 24 and 48 h as indicated. The RNA samples were analyzed by RT-PCR. rp49 was used as the experimental expression standard.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download