Abstract
Epstein-Barr virus (EBV) latent infection transforms B lymphocytes into proliferating lymphoblastoid cell lines (LCLs). EBV latent infection membrane protein 1 (LMP1) is required for EBV-mediated B lymphocyte transformation, and LMP1-induced NF-κB activation is essential for LCL survival. Previously, it was reported that the level of reactive oxygen species (ROS) and the expression of apoptosis signal-regulating kinase 1 (ASK1) are elevated in EBV-positive Burkitt's lymphoma (BL) cells, the potential role of ASK1 in LMP1-induced NF-κB activation was thus investigated in this study. In EBV-positive BL cells, ASK1 was highly expressed and activated. In addition, TRAF6-ASK1 interaction was significantly increased in EBV-positive BL cells. Interestingly, the expression of LMP1 alone facilitated ASK1 activation. The expression of a dominant negative ASK1 mutant (ASK1KM) strongly blocked LMP1-induced NF-κB activation. Furthermore, LMP1-induced NF-κB activation was significantly reduced in ASK1 knock out (ASK1-/-) mouse embryonic fibroblasts (MEFs). Taken together, these results demonstrate that ASK1 is activated by LMP1 and is critical for LMP1-induced NF-κB activation.
Epstein Barr Virus (EBV), a member of the human γ-herpesvirus family, is one of the most common viruses in human and is causally associated with lymphoid and epithelial malignancies such as Burkitt's lymphoma (BL), T-cell lymphoma, Hodgkin's Disease, and Nasopharyngeal Carcinoma (1, 2). Latent-infection membrane protein 1 (LMP1), expressed in most EBV-associated malignancies, is an EBV oncogenic protein that is essential for EBV-mediated primary B lymphocyte transformation into proliferating lymphoblastoid cell lines (LCLs) (1, 2). LMP1 is composed of a 24-amino acids (aa) cytosolic N-terminus, 162-aa six hydrophobic transmembrane domains and a 200-aa cytosolic C-Terminus (reviewed in (3)). Using the transmembrane domains, LMP1 aggregates in plasma membrane lipid rafts and constitutively activates NF-κB, p38 mitogen-activated protein kinase (MAPK), c-Jun N-terminal kinase (JNK), and interferon regulatory factor 7 (IRF7) through two C-terminal cytoplasm signaling domains referred to as C-Terminal Activation Regions (CTAR) 1 and 2 (4~11). LMP1-mediated NF-κB activation is essential for EBV-transformed LCL survival (12, 13).
NF-κB is a transcription factor family regulating expression of the genes involved in cell proliferation, differentiation, and survival (reviewed in (14, 15)). The NF-κB family consists of p65/RelA, RelB, c-Rel, p105/p50 and p100/p52 and forms homo-or hetero-dimers to transactivate various genes (14~16). There are two types of NF-κB activation pathways. One is a canonical pathway for NF-κB activation that involves the p65/p50 complexes that are retained in the cytoplasm by inhibitor of κB (IκB) proteins. In response to various stimuli, IκB proteins are phosphorylated by IκB kinase β (IKKβ) and degraded by the ubiquitin-proteasome pathway allowing the p65/p50 complexes to translocate into the nucleus. The other is an alternative pathway for NF-κB activation that involves NF-κB inducing kinase (NIK)-and IKKα-mediated proteolytic processing of p100 into p52 and translocation of the RelB/p52 complexes into the nucleus (14, 15). LMP1 can activate both the canonical and the alternative pathways for NF-κB activation (14, 15). LMP1 CTAR1 engages TRAF1, 2, 3, and 5 through a consensus PXQXT motif found in the CD40 and activates the alternative pathway for NF-κB activation. CTAR2 engages TNFR-associated death domain protein (TRADD) and receptor rnteracting protein kinase 1 (RIP1) leading to the activation of the canonical pathway for NF-κB activation (17~21).
Apoptosis signal-regulating kinase 1 (ASK1) is a MAPK kinase kinase (MAP3K) that is activated in response to cytotoxic stresses, and proinflammatory cytokines such as interlueukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), lipopolysaccharide (LPS), reactive oxygen species (ROS), endoplasmic reticulum (ER) stress and calcium influx (16, 22, 23). In response to these stimuli, ASK1 activates p38 MAPK and JNK, thereby regulating various biological responses which include cell death by apoptosis, inflammation, differentiation, and survival (24).
EBV-positive Burkitt's lymphoma (BL) cells exhibit elevated levels of ROS (25), and the expression of EBV nuclear antigen 2 (EBNA2) or LMP1 alone can induce ROS generation (25). Interestingly, ROS is essential for NF-κB activation, but not for MAPK activation, in EBV-positive BL cells (25). Therefore, in the present study, we investigated the role of ASK1 in EBV LMP1-induced NF-κB activation.
Maintenance and propagation of HEK293, HeLa, EBV-negative Burkitt's lymphoma BL41, and BL41 cells infected with the B958 strain of EBV (BL41-B958) have been described previously (13, 26). ASK1 knock-out (KO) mouse embryonic fibroblasts (MEFs), pCDNA3-HA-ASK1 (WT), and pCDNA3-HA-ASK1KM (K709M) were kindly provided by Dr. Hidenori Ichijo (The University of Tokyo). pSG5-FLAG-LMP1 was previously described (27). For transient transfection, Effectene transfection reagents were used according to the manufacturer's directions (Qiagen, Valencia, CA, USA). Luciferase assays were performed as previously described (28). Luciferase data shown here represent the mean values of three independent experiments.
Cells were collected, fractionated, and transferred to nitrocellulose membranes as described previously (29). Antibodies to HA, ASK1, IRAK1, and TRAF6 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibody to phospho-ASK1 Thr845 was purchased from Cell Signaling Technology (Beverly, MA, USA). Antibody to alpha-tubulin was purchased from Sigma Aldrich (St. Louis, MO, USA). Enhanced chemiluminescence detection reagents (Pierce, Rockford, IL, USA) and secondary peroxidase-labeled anti-mouse or anti-rabbit immunoglobulin G antibody (Amersham Biosciences, Piscataway, NJ, USA) were used according to the manufacturer's directions.
Ten million cells were lysed in buffer containing 20 mM Tris-HCl [pH 7.5], 100 mM NaCl, 0.5 mM EDTA, 1% NP-40, phosphatase inhibitor cocktail (Calbiochem, La Jolla, CA, USA), and protease inhibitor cocktail (Roche, Indianapolis, IN). Lysates were pre-cleared with protein A/G agarose beads (Santa Cruz Biotechnology) and incubated at 4℃ for overnight with anti-TRAF6 antibody (Santa Cruz Biotechnology). After washing three times with lysis buffer, protein complexes were analyzed by Western blot.
Since EBV-positive BL cell was associated with high levels of ROS (25), the status of ASK1 expression and activation in EBV-positive BL41-B958 cells was determined (Fig. 1). As previously reported (13), the expression of ASK1 was strongly induced in EBV-positive BL41-B958 cells compared to EBV-negative BL41 cells (Fig. 1, compare lane 2 with lane 1). In addition, phosphorylation of threonine 845 in the activation loop of ASK1 was significantly increased in BL41-B958 cells (Fig. 1, compare lane 2 with lane 1). These data indicate that ASK1 is highly expressed and activated in EBV-positive BL41-B958 cells.
Since ROS induced the interaction between TRAF6 and ASK1 (16), we further determined the interaction of TRAF6 to ASK1 in EBV-positive BL cell lines. The lysates of BL41 cells, BL41 cells treated with IL-1β, or BL41-B958 cells were immunoprecipitated with anti-TRAF6 antibody, and ASK1 binding was assessed by Western blot (Fig. 2). In BL41 cells treated with IL-1β or BL41-B958 cells, the interaction between TRAF6 and ASK1 was significantly increased (Fig. 2, compare lanes 1 and 3 with lane 2). In addition, TRAF6 interaction with IRAK1 was also higher in BL41 cells treated with IL-1β or BL41-B958 cells (Fig. 2, compare lanes 1 and 3 with lane 2). Thus, the interaction between TRAF6 and ASK1 or IRAK1 is strongly induced in EBV-positive BL41-B958 cells compared to EBV-negative BL41 cells.
Since LMP1 induces ROS (25), we further investigated the role of LMP1 in ASK1 activation (Fig. 3). HeLa cells were transfected with either pSG5 or pSG5-FLAG-LMP1 plus pcDNA3-HA-ASK1, and cell lysates were subjected to Western blot analysis with anti-phospho-ASK1 Thr845 antibody, anti-HA antibody, or anti-LMP1 antibody at 24 h after transfection. Interestingly, ASK1 phosphorylation at threonine 845 was strongly induced in cells transfected with pSG5-FLAG-LMP1, but not in cells transfected with pSG5 (Fig. 3, compare lane 2 with lane 1). These data indicate that LMP1 activates ASK1 in HeLa cells.
ROS inhibition significantly reduces NF-κB activation in EBV-positive BL cells (25), and ASK1 has been reported to play an important role in NF-κB activation (14, 15). To assess the role of ASK1 in LMP1-induced NF-κB activation, a dominant negative ASK1 mutant (ASK1KM) was overexpressed in HEK293 cells, and NF-κB activation was determined using an NF-κB-dependent luciferase reporter assay (Fig. 4A). Interestingly, increasing amounts of ASK1KM strongly interfered with LMP1-induced NF-κB activation (Fig. 4A, compare lane 2 with lanes 4 and 6). To further investigate the role of ASK1 in LMP1-induced NF-κB activation, ASK1 wild-type (ASK1+/+) or knock-out (ASK1-/-) MEFs were transfected with either pSG5 or pSG5-FLAG-LMP1, and NF-κB activation was determined using an NF-κB-dependent luciferase reporter assay (Fig. 4B). In ASK1 knock-out MEFs, LMP1-induced NF-κB activation was reduced by 75% compared to ASK1 wild-type MEFs (Fig. 4B, compare lane 4 with lane 2). These data indicate that ASK1 plays a significant role in LMP1-induced NF-κB activation.
LMP1 is essential for EBV-infected B lymphocyte transformation to proliferating LCLs and functionally mimics CD40, a member of the tumor necrosis factor receptor (TNFR) superfamily (1). Since LMP1-induced NF-κB activation is critical for EBV-transformed LCL survival (12, 13), delineation of LMP1-induced NF-κB activation pathway may provide a potential drug target in EBV-associated cancers.
Recently, it was reported that the levels of ROS are significantly elevated in EBV-positive BL cells, and ROS is not essential for MAPK activation but contributes to NF-κB activation (25). Since LMP1 alone can induce ROS generation (25), we investigated whether LMP1 activates ASK1 and whether ASK1 is critical for LMP1-induced NF-κB activation.
The data presented here indicate that ASK1 is highly expressed and activated in EBV-positive BL cells, and LMP1 alone can activate ASK1 in HeLa cells. ROS activates TRAF6-ASK1-p38 MAPK pathway that is essential for Toll-like receptor 4 (TLR4) mediated innate immune responses (16). Similar to the TLR4 signaling pathway, TRAF6 is strongly associated with ASK1 or IRAK1 in EBV-positive BL cells. Although there were several reports indicating that ASK1 may be important for NF-κB activation (14, 15), ASK1 has been thought to be essential for p38 MAPK and JNK, but not for NF-κB activation. However, our experiments using both dominant negative ASK1 mutants and ASK1 knock-out MEFs provide strong evidence that ASK1 is required for LMP1-induced NF-κB activation.
Taken together, the results of this study demonstrate that ASK1 is activated by LMP1 and is critical for LMP1-induced NF-κB activation. ASK1 may also play an important role in LMP1-induced p38 MAPK or JNK activation. How LMP1 activates ASK1 and whether ROS or ASK1 is essential for LCL survival are unclear and are the subjects of future studies.
Figures and Tables
Figure 1
ASK1 is activated in BL41-B958 cells. Equal amounts of BL41 or BL41-B958 cell extracts were subjected to Western blot analysis with anti-ASK1, anti-phopho-ASK1 Thr845 (P-ASK1), anti-LMP1, or anti-tubulin antibody.

Figure 2
TRAF6-ASK1 or TRAF6-IRAK1 interaction is induced in BL41-B958 cells. BL41 or BL41-B958 cells were lysed, and each cell lysate was immunoprecipitated with anti-TRAF6 antibody. The immunoprecipitates were subjected to Western blot analysis with anti-ASK1, anti-IRAK1 or anti-TRAF6 antibody.
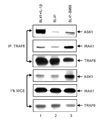
Figure 3
LMP1 activates ASK1. HeLa cells were transfected with either pSG5 or pSG5-FLAG-LMP1 plus pcDNA3-HA-ASK1. After 24 h, equal amounts of cell extracts were subjected to Western blot analysis with anti-phospho-ASK1 Thr845 antibody (P-ASK1), anti-HA antibody, or anti-LMP1 antibody.

Figure 4
ASK1 plays an important role in LMP1-induced NF-κB activation. (A) HEK293 cells were transfected with either pSG5 (lanes 1, 3 and 5) or pSG5-FLAG-LMP1 (lanes 2, 4 and 6) with increasing amounts of ASK1KM (lanes 3 to 6). At 24 h after transfection, LMP1-induced NF-κB activation was determined using an NF-κB-dependent luciferase reporter assay. LMP1 protein levels were determined by Western blot analysis with anti-LMP1 antibody. (B) ASK1 wild-type (ASK1+/+) or knock-out (ASK1-/-) mouse embryonic fibroblasts (MEFs) were transfected with either pSG5 or pSG5-FLAG-LMP1, and then NF-κB activation was determined using an NF-κB-dependent luciferase reporter assay at 24 h after transfection. LMP1 protein levels were determined by Western blot analysis with anti-LMP1 antibody.

References
1. Kieff ED, Rickinson AB. Knipe DM, Howley PM, editors. Epstein-Barr Virus and Its Replication. Fields Virology. 2007. Philadelphia: Lippincott, Williams, and Wilkins;2603–2655.
2. Rickinson AB, Kieff ED. Knipe DM, Howley PM, editors. Epstein-Barr Virus. Fields Virology. 2007. Fifth ed. Philadelphia, Pa: Lippincott, Williams, and Wilkins;2655–2700.
3. Li HP, Chang YS. Epstein-Barr virus latent membrane protein 1: structure and functions. J Biomed Sci. 2003. 10:490–504.

4. Mitchell T, Sugden B. Stimulation of NF-kappa B-mediated transcription by mutant derivatives of the latent membrane protein of Epstein-Barr virus. J Virol. 1995. 69:2968–2976.

5. Huen DS, Henderson SA, Croom-Carter D, Rowe M. The Epstein-Barr virus latent membrane protein-1 (LMP1) mediates activation of NF-kappa B and cell surface phenotype via two effector regions in its carboxy-terminal cytoplasmic domain. Oncogene. 1995. 10:549–560.
6. Devergne O, Cahir McFarland ED, Mosialos G, Izumi KM, Ware CF, Kieff E. Role of the TRAF binding site and NF-kappaB activation in Epstein-Barr virus latent membrane protein 1-induced cell gene expression. J Virol. 1998. 72:7900–7908.

7. Devergne O, Hatzivassiliou E, Izumi KM, Kaye KM, Kleijnen MF, Kieff E, et al. Association of TRAF1, TRAF2, and TRAF3 with an Epstein-Barr virus LMP1 domain important for B-lymphocyte transformation: role in NF-kappaB activation. Mol Cell Biol. 1996. 16:7098–7108.

8. Mosialos G, Birkenbach M, Yalamanchili R, VanArsdale T, Ware C, Kieff E. The Epstein-Barr virus transforming protein LMP1 engages signaling proteins for the tumor necrosis factor receptor family. Cell. 1995. 80:389–399.

9. Kaye KM, Devergne O, Harada JN, Izumi KM, Yalamanchili R, Kieff E, et al. Tumor necrosis factor receptor associated factor 2 is a mediator of NF-kappa B activation by latent infection membrane protein 1, the Epstein-Barr virus transforming protein. Proc Natl Acad Sci U S A. 1996. 93:11085–11090.

10. Kaye KM, Izumi KM, Mosialos G, Kieff E. The Epstein-Barr virus LMP1 cytoplasmic carboxy terminus is essential for B-lymphocyte transformation; fibroblast cocultivation complements a critical function within the terminal 155 residues. J Virol. 1995. 69:675–683.

11. Izumi KM, Cahir McFarland ED, Riley EA, Rizzo D, Chen Y, Kieff E. The residues between the two transformation effector sites of Epstein-Barr virus latent membrane protein 1 are not critical for B-lymphocyte growth transformation. J Virol. 1999. 73:9908–9916.

12. Cahir-McFarland ED, Davidson DM, Schauer SL, Duong J, Kieff E. NF-kappa B inhibition causes spon taneous apoptosis in Epstein-Barr virus-transformed lymphoblastoid cells. Proc Natl Acad Sci U S A. 2000. 97:6055–6060.

13. Cahir-McFarland ED, Carter K, Rosenwald A, Giltnane JM, Henrickson SE, Staudt LM, et al. Role of NF-kappa B in cell survival and transcription of latent membrane protein 1-expressing or Epstein-Barr virus latency III-infected cells. J Virol. 2004. 78:4108–4119.

14. Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002. 109:S81–S96.
15. Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004. 18:2195–2224.
16. Matsuzawa A, Saegusa K, Noguchi T, Sadamitsu C, Nishitoh H, Nagai S, et al. ROS-dependent activation of the TRAF6-ASK1-p38 pathway is selectively required for TLR4-mediated innate immunity. Nat Immunol. 2005. 6:587–592.

17. Eliopoulos AG, Gallagher NJ, Blake SM, Dawson CW, Young LS. Activation of the p38 mitogen-activated protein kinase pathway by Epstein-Barr virus-encoded latent membrane protein 1 coregulates interleukin-6 and interleukin-8 production. J Biol Chem. 1999. 274:16085–16096.

18. Eliopoulos AG, Blake SM, Floettmann JE, Rowe M, Young LS. Epstein-Barr virus-encoded latent membrane protein 1 activates the JNK pathway through its extreme C terminus via a mechanism involving TRADD and TRAF2. J Virol. 1999. 73:1023–1035.

19. Eliopoulos AG, Stack M, Dawson CW, Kaye KM, Hodgkin L, Sihota S, et al. Epstein-Barr virus-encoded LMP1 and CD40 mediate IL-6 production in epithelial cells via an NF-kappaB pathway involving TNF receptor-associated factors. Oncogene. 1997. 14:2899–2916.

20. Eliopoulos AG, Dawson CW, Mosialos G, Floettmann JE, Rowe M, Armitage RJ, et al. CD40-induced growth inhibition in epithelial cells is mimicked by Epstein-Barr Virus-encoded LMP1: involvement of TRAF3 as a common mediator. Oncogene. 1996. 13:2243–2254.
21. Kieser A, Kilger E, Gires O, Ueffing M, Kolch W, Hammerschmidt W. Epstein-Barr virus latent membrane protein-1 triggers AP-1 activity via the c-Jun N-terminal kinase cascade. EMBO J. 1997. 16:6478–6485.

22. Matsukawa J, Matsuzawa A, Takeda K, Ichijo H. The ASK1-MAP kinase cascades in mammalian stress response. J Biochem. 2004. 136:261–265.

23. Takeda K, Matsuzawa A, Nishitoh H, Tobiume K, Kishida S, Ninomiya-Tsuji J, et al. Involvement of ASK1 in Ca2+-induced p38 MAP kinase activation. EMBO Rep. 2004. 5:161–166.

24. Takeda K, Matsuzawa A, Nishitoh H, Ichijo H. Roles of MAPKKK ASK1 in stress-induced cell death. Cell Struct Funct. 2003. 28:23–29.

25. Cerimele F, Battle T, Lynch R, Frank DA, Murad E, Cohen C, et al. Reactive oxygen signaling and MAPK activation distinguish Epstein-Barr Virus (EBV)-positive versus EBV-negative Burkitt's lymphoma. Proc Natl Acad Sci U S A. 2005. 102:175–179.

26. Stinski MF. Synthesis of proteins and glycoproteins in cells infected with human cytomegalovirus. J Virol. 1977. 23:751–767.

27. Song YJ, Izumi KM, Shinners NP, Gewurz BE, Kieff E. IRF7 activation by Epstein-Barr virus latent membrane protein 1 requires localization at activation sites and TRAF6, but not TRAF2 or TRAF3. Proc Natl Acad Sci U S A. 2008. 105:18448–18453.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download