Abstract
We have developed the recombinant adeno-associated virus (AAV) carrying the EGFP gene under the control of the microphtalmia-associated transcription factor-M (MITF-M) promoter region for melanoma-specific expression. MITF-M distal enhancer (MDE) region enhances the specific expression of the reporter gene specifically in cultured melanoma cells. Expression of EGFP protein was very high in AAV-CMV-EGFP infected cells but relatively low in cells infected with AAV-Mitf(Enh/Pro)-EGFP. After an in vitro infection by a recombinant AAV carrying the EGFP gene under the control of human MITF-M promoter, the reporter gene was expressed in MITF-M producing melanoma cell lines (SK-28 and G361), but not in MITF-M non-producing cell lines (HaCat). These results suggest that the utilization of the MITF-M promoter in a recombinant AAV vector could provide benefits in gene therapy applications.
Among the vectors which can be considered for therapeutic application to skin, adeno-associated virus (AAV) seems to possess several appealing features, including the capacity to promote long-term expression of the therapeutic gene in the absence of signs of inflammation or immune response (1). These vectors are derived from a non-pathogenic and widespread defective parvovirus, and are able to transduce dividing or non-dividing cells, including skeletal and cardiac muscle (2, 3), brain (4) and liver (5).
Microphtalmia-associated transcription factor-M (MITF-M) is essential for differentiation of melanoblasts to melanocytes and contains a basic helix-loop-helix leucine zipper (bHLH-LZ) structure (6, 7). This gene is exclusively expressed in melanocytes and melanoma cells, and is under the control of the MITF-M promoter (7~9). Fuse et al. (1996) published that the segment (position -387 to transcription initiation site) contains the putative promoter region (9). A TATA-like sequence is located at position -21. Within the promoter region, there are some potential cis-acting elements [positions -196 to -191 and -8 to -3] (10), a cyclic AMP-response element [-147 to -140] (11), and an interleukin-6-responsive element [-258 to -277] (12). Watanabe et al. (2002) have identified the 298-bp MITF-M distal enhancer (MDE) that promotes pigment cell-specific transcription from the MITF-M promoter (7).
In this study, we constructed recombinant AAV carrying the EGFP gene under the control of the MITF-M promoter region for melanoma-specific expression. The enhancer and promoter region resulted in melanocyte-specific expression of the reporter gene specifically in cultured melanoma cells. We showed that the control of the melanocyte-specific promoter in the recombinant AAV significantly increase the specificity of expression of human melanoma cell lines.
Taq DNA polymerase and the AAV helper free system were purchased from Promega (Madison, WI) and Stratagene (La Jolla, CA). The human skin melanocyte cells were purchased from Cascade Biologics (Portland, OR). The human immortalized keratinocyte cell (HaCat) and melanoma cells (SK-28 and G361) were a gift from Young-Il Kim, Kyunghee University, South Korea. The cells were grown in Dulbecco's modified Eagle medium (DMEM; GIBCO-BRL, Grand Island, NY) or in RPMI1640 supplemented with 10% FBS and 4 mM glutamine. Cultures were maintained at 37℃ in a humidified atmosphere of 95% air/5% CO2.
The genomic DNA was isolated from the human skin melanocyte cells using the TRIZOL reagent (Invitrogen, NY) according to the manufacturer's instructions. The amount of genomic DNA was measured spectrophotometrically by the absorbance of 260 nm and stored at -20℃ until use.
The 500-bp human MITF-M promoter region was amplified using primers Mpro-F (5'-CTG CAG TCG GAA GTG GCA GTT ATT C-3') and Mpro-R (5'-GCT AGC CGC GGG TAG ACT TAT CCC TCC CTC TAC-3'). A 298-bp enhancer fragment was amplified with primer pair Menh-F (5'-GGT ACC ACG CGT GAT TAC AGG AGT GAG CCA CCA CA-3') and Menh-R (5'-GCT AGC TAT CTG CAG CTT CCA GAC TTC AAT GGC AAT-3'). The programs for PCR amplification with each primer set were as follows: 1 cycle at 95℃ for 5 min followed by 50 cycles at 94℃ for 1 min, 56℃ for 2 min, and 72℃ for 1 min with final 1 cycle at 72℃ for 10 min for MITF-M enhancer and promoter regions. Each PCR fragments were cloned into the pCR2.1-TOPO plasmid. The resulting plasmids are termed as pCR2.1-MITFenh and pCR2.1-MITFprp, respectively. The recombinant AAV plasmid pAAV-EGFP was generated by inserting expression cassette encoding the enhanced green fluorescence protein (EGFP) reporter gene into the standard AAV transfer vector pAAV. First, the promoter gene was excised as about 500-bp KpnI/NheI fragment from the promoter plasmid DNA (pCR2.1-MITFp). This fragment was inserted into the same sites of enhancer plasmid DNA (pCR2.1-MITFe) in a direct orientation with respect to the enhancer to create pCR2.1-MITFe/p plasmid. In order to create pAAV-Mitf(Enh/Pro)-EGFP plasmid, a 800-bp enhancer/promoter fragment of MITF-M gene was prepared by MluI/KspI double digestion, and was inserted into the same sites of pAAV-EGFP.
The recombinant AAVs were generated according to the manufacturer's instructions. The titer varied from 1 to 3 × 102 plaque-forming units per milliliter (data not shown). Approximately 1 × 105 HaCat, SK-28 and G361 cells were plated in each well of 6-well plate and incubated at 37℃ for 24 h. Cells were washed once with complete medium and then infected at 37℃ for 1 h with mock and 1 × 102 particles per cell of recombinant AAV-CMV or AAV-Mitf (Enh/Pro)-EGFP as described previously (13). Cells were incubated in complete medium for 4 or 5 days. The transduction efficiency was measured by EGFP imaging using a NIKON fluorescence microscope (Nikon, Inc., Japan).
The pAAV-CMV-EGFP plasmid DNA was generated by inserting expression cassette encoding the EGFP reporter gene into the standard AAV transfer vector pAAV-MCS in an orientation such that the direction of transcription was convergent (Fig. 1). Next, the recombinant pAAV-Mitf(Enh/Pro)-EGFP was generated by inserting melanocyte-specific enhancer/promoter region into the pAAV-CMV-EGFP plasmid instead of CMV promoter. The EGFP gene under the control of CMV promoter is highly expressed in all infected cells, allowing easy detection of viral plaques. The replacement of CMV promoter with MITF-M enhancer/promoter drives melanocyte-specific gene expression. The AAV-293 cell line was used to generate recombinant AAV.
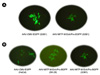
Fig. 2A shows a comparison of EGFP expression in AAV-CMV-EGFP or AAV-Mitf(Enh/Pro)-EGFP infected melanoma cells. EGFP expression was robust in cells infected with AAV-CMV-EGFP but relatively weak in cells infected with AAV-Mitf(Enh/Pro)-EGFP.
AAV-mediated EGFP expression was first observed at 4 or 5 days after infection and last up to 7 days post infection (Data not shown). The MITF-M gene is normally expressed in melanocyte but transcriptionally silent in other tissues, and also, it can be normally expressed in melanoma. Two human melanoma cell lines, SK-28 and G361, were used as target cells for recombinant AAV infection. To analyze the effect of MITF-M promoter on expression of the transfer gene, two melanoma cell lines were infected with AAV-Mitf(Enh/Pro)-EGFP and AAV-CMV-EGFP. At an m.o.i. of 10, EGFP expression was detected in two melanoma cell (Fig. 2B, SK-28 and G361). In contrast, EGFP protein was not detected in MITF-M no producing cell lines (HaCat), even at a same m.o.i.. Therefore, it was concluded that EGFP was expressed only in melanoma cells.
Up to the present, various types of gene therapy for cancer have been developed. Retrovirus system has an advantage of mediating stable gene transfer with a low potential for immunogenicity (14, 15), but the vector delivery system has the limitation for in vivo usage (16, 17). These include the difficulties in producing high-titer virus, the fact that only dividing cells are infected, and the possibility of insertional mutagenesis. On the other hand, adenoviral vectors deliver genes to the liver at very high efficiencies, approaching 100% gene transduction to hepatocytes (18). However, the major disadvantages of adenoviral vectors are the instability of the transferred genes in the target cells due to a lack of integration and the induction of immunological responses (19, 20). As an alternative gene-delivery system, recombinant AAV constitute one of the leading vectors used for regulated promoters of gene delivery (21). Other groups have reported the use of regulated promoters containing tetracycline (22~25), rapamycin (26~28), and tissue-specific promoters (21). The recombinant AAV has been shown to be an ideal for gene transferring and expressing foreign genes in mammalian cells, stable integration and long-term expression, safety, and the option of using large constructs (21).
In this study, we have generated recombinant AAV carrying MITF-M enhancer and promoter region by cloning the MITF-M enhancer and promoter genes in the AAV genome. Using MITF-M producing SK-28 and G361 cell lines and MITF-M non-producing human keratinocyte cell, HaCat, it was observed that EGFP was expressed only in the melanoma cells. This vector presented one serious problem; nonspecific transduction of heterologous gene of interest into cells other than target cells. To circumvent this problem, we take advantage of the selectivity of melanocyte-type promoter for melanoma cells. Thus, this promoter selectivity allowed high-level trans-gene production in melanoma cells.
Our study indicates that the reporter gene could be efficiently transferred to human melanoma cells, using recombinant AAV under the control of the MITF-M promoter. It was concluded that the heterologous gene of interest could be efficiently transferred to human MITF-M-producing melanoma cells using recombinant AAV-Mitf (Enh/Pro)-EGFP in vitro.
Figures and Tables
Figure 1
Cloning of the recombinant AAV transfer vectors. (A) pAAV-MCS plasmid DNA vector contained multi cloning sites under the control of the CMV promoter. The gene cassette was terminated by a bovine growth hormone (bGH) poly (A) addition site. (B) The pAAV-CMV-EGFP plasmid DNA was generated by inserting expression cassette encoding the EGFP reporter gene into the standard AAV transfer vector pAAV-MCS. (C) Next, the recombinant pAAV-Mitf(Enh/Pro)-EGFP DNA was generated by inserting melanocyte-specific enhancer/promoter region into the pAAV-CMV-EGFP plasmid instead of CMV promoter.

Figure 2
Cell type specific gene expression by MITF-M enhancer/promoter. (A) EGFP protein was very highly expressed in AAV-CMV-EGFP infected cells but relatively low detected in cells infected with AAV-Mitf(Enh/Pro)-EGFP. (B) Two melanoma cell lines were infected with AAV-Mitf(Enh/Pro)-EGFP and AAV-CMV-EGFP at an m.o.i. of 10. The EGFP activity was detected in two melanoma cells (SK-28 and G361). However, EGFP gene expression was not detected in MITF-M no producing cell line (HaCat). Cells were photographed by epifluorescence microscopy at maximum expression point.
References
1. Galeano M, Deodato B, Altavilla D, Cucinotta D, Arsic N, Marini H, et al. Adeno-associated viral vector-mediated human vascular endothelial growth factor gene transfer stimulates angiogenesis and wound healing in the genetically diabetic mouse. Diabetologia. 2003. 46:546–555.

2. Snyder RO, Spratt SK, Lagarde C, Bohl D, Kaspar B, Sloan B, et al. Efficient and stable adeno-associated virus-mediated transduction in the skeletal muscle of adult immunocompetent mice. Hum Gene Ther. 1997. 8:1891–1900.

3. Su H, Lu R, Kan YW. Adeno-associated viral vector-mediated vascular endothelial growth factor gene transfer induces neovascular formation in ischemic heart. Proc Natl Acad Sci U S A. 2000. 97:13801–13806.

4. Kaplitt MG, Leone P, Samulski RJ, Xiao X, Pfaff DW, O'Malley KL, et al. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat Genet. 1994. 8:148–154.

5. Xiao W, Berta SC, Lu MM, Moscioni AD, Tazelaar J, Wilson JM. Adeno-associated virus as a vector for liver-directed gene therapy. J Virol. 1998. 72:10222–10226.

6. Yajima I, Sato S, Kimura T, Yasumoto K, Shibahara S, Goding CR, et al. An L1 element intronic insertion in the black-eyed white (Mitf[mi-bw]) gene: the loss of a single Mitf isoform responsible for the pigmentary defect and inner ear deafness. Hum Mol Genet. 1999. 8:1431–1441.

7. Watanabe K, Takeda K, Yasumoto K, Udono T, Saito H, Ikeda K, et al. Identification of a distal enhancer for the melanocyte-specific promoter of the MITF gene. Pigment Cell Res. 2002. 15:201–211.

8. Amae S, Fuse N, Yasumoto K, Sato S, Yajima I, Yamamoto H, et al. Identification of a novel isoform of microphthalmia-associated transcription factor that is enriched in retinal pigment epithelium. Biochem Biophys Res Commun. 1998. 247:710–715.

9. Fuse N, Yasumoto K, Suzuki H, Takahashi K, Shibahara S. Identification of a melanocyte-type promoter of the microphthalmia-associated transcription factor gene. Biochem Biophys Res Commun. 1996. 219:702–707.

10. Evans T, Reitman M, Felsenfeld G. An erythrocyte-specific DNA-binding factor recognizes a regulatory sequence common to all chicken globin genes. Proc Natl Acad Sci U S A. 1988. 85:5976–5980.

11. Montminy MR, Sevarino KA, Wagner JA, Mandel G, Goodman RH. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci U S A. 1986. 83:6682–6686.

12. Oliviero S, Cortese R. The human haptoglobin gene promoter: interleukin-6-responsive elements interact with a DNA-binding protein induced by interleukin-6. EMBO J. 1989. 8:1145–1151.

13. Qing K, Hansen J, Weigel-Kelley KA, Tan M, Zhou S, Srivastava A. Adeno-associated virus type 2-mediated gene transfer: role of cellular FKBP52 protein in transgene expression. J Virol. 2001. 75:8968–8976.

15. Dachs GU, Dougherty GJ, Stratford IJ, Chaplin DJ. Targeting gene therapy to cancer: A review. Oncol Res. 1997. 9:313–325.
16. Baum C, Eckert HG, Stockschläder M, Just U, Hegewisch-Becker S, Hildinger M, et al. Improved retroviral vectors for hematopoietic stem cell protection and in vivo selection. J Hematother. 1996. 5:323–329.

17. Dunbar CE, Tisdale J, Yu JM, Soma T, Zujewski J, Bodine D, et al. Transduction of hematopoietic stem cells in humans and in nonhuman primates. Stem Cells. 1997. 15:135–139. discussion 139-40.

18. Li Q, Kay MA, Finegold M, Stratford-Perricaudet LD, Woo SL. Assessment of recombinant adenoviral vectors for hepatic gene therapy. Hum Gene Ther. 1993. 4:403–409.

19. Engelhardt JF, Ye X, Doranz B, Wilson JM. Ablation of E2A in recombinant adenoviruses improves transgene persistence and decreases inflammatory response in mouse liver. Proc Natl Acad Sci U S A. 1994. 91:6196–6200.

20. Park SW, Lee HK, Kim TG, Yoon SK, Paik SY. Hepatocyte-specific gene expression by baculovirus pseudotyped with vesicular stomatitis virus envelope glycoprotein. Biochem Biophys Res Commun. 2001. 289:444–450.

21. Gafni Y, Pelled G, Zilberman Y, Turgeman G, Apparailly F, Yotvat H, et al. Gene Therapy Platform for Bone Regeneration Using an Exogenously Regulated, AAV-2-Based Gene Expression System. Mol Ther. 2004. 9:587–595.

22. Haberman RP, McCown TJ, Samulski RJ. Inducible long-term gene expression in brain with adeno-associated virus gene transfer. Gene Ther. 1998. 5:1604–1611.

23. Fitzsimons HL, Mckenzie JM, During MJ. Insulators coupled to a minimal bidirectional tet cassette for tight regulation of rAAV-mediated gene transfer in the mammalian brain. Gene Ther. 2001. 8:1675–1681.

24. McGee Sanftner LH, Rendahl KG, Quiroz D, Coyne M, Ladner M, Manning WC, et al. Recombinant AAV-mediated delivery of a tet-inducible reporter gene to the rat retina. Mol Ther. 2001. 3:688–696.

25. Chtarto A, Bender HU, Hanemann CO, Kemp T, Lehtonen E, Levivier M, et al. Tetracycline-inducible transgene expression mediated by a single AAV vector. Gene Ther. 2003. 10:84–94.

26. Angeletti B, Löster J, Auricchio A, Gekeler F, Shinoda K, Ballabio A, et al. An in vivo doxycycline-controlled expression system for functional studies of the retina. Invest Ophthalmol Vis Sci. 2003. 44:755–760.

27. Haberman RP, McCown TJ. Regulation of gene expression in adeno-associated virus vectors in the brain. Methods. 2002. 28:219–226.

28. Johnston J, Tazelaar J, Rivera VM, Clackson T, Gao GP, Wilson JM. Regulated expression of erythropoietin from an AAV vector safely improves the anemia of beta-thalassemia in a mouse model. Mol Ther. 2003. 7:493–497.

29. Yang YW, Kotin RM. Glucose-responsive gene delivery in pancreatic islet cells via recombinant adeno-associated viral vectors. Pharm Res. 2000. 17:1056–1061.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download