Abstract
Bartonellosis is spotlighted recently as an emerging zoonosis and Bartonella henselae is reported to be the main infectious agent. In Korea, however, few studies have been made on the epidemiology and microbiology on bartonellosis. Thus, this study was conducted to produce a new monoclonal antibody that can be used for identifying B. henselae. In order to prepare monoclonal antibodies against B. henselae, we inoculated mice with the isolated strain from Korean patient and performed cell fusion experiment. The selected hybridoma clones produced monoclonal antibodies which showed positive immunofluorescence staining of bacteria and specific protein bands in western blot analysis. In order to examine whether these antibodies could be used for the identifying and quantifying Bartonella, we performed confocal microscopy and flow cytometry using the new antibodies. These monoclonal antibodies can be used as a useful tool in further researches on the biology of Bartonella.
Figures and Tables
 | Figure 1The immunofluorescence antibody test of hybridoma clones secreting the monoclonal antibodies against Bartonella. Culture supernatant of hybridoma clones were tested for their reactivity against B. henselae that was included in the commercial antibody test kit. |
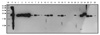 | Figure 2Western blot analysis of the 24 hybridoma clones secreting the monoclonal antibodies against Bartonella. The Korean isolate of B. henselae was grown in blood agar plate and harvested. After separation by SDS-PAGE, the antigenic bands were transferred to polyvinylidene difluoride membrane and cut into small strips. Each strip was analyzed using the supernatants of the hybridoma clones as a primary antibody. M, Protein size marker; P, positive control (B. henselae infected mouse serum); N, negative control (supernatant of the V653 cell cultured media); lane 1~28, the culture supernatants of each hybridoma clones. |
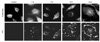 | Figure 3The reactivity of a monoclonal antibody against ECV 304 cells infected with B. henselae was examined with the confocal microscope. The culture supernatant of a selected hybridoma clone (#1) was used as a primary antibody and the results were analyzed using the confocal laser scanning microscopy at the indicated times (0, 1, 3, 6 and 24 h after infection). ECV cell bodies were stained with Evans blue and bacterial cells were stained with IFA. |
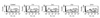 | Figure 4The reactivity of a monoclonal antibody against ECV 304 cells infected with B. henselae was examined with flow cytometry. The culture supernatant of a selected hybridoma clone (#1) was used as a primary antibody and the results were analyzed using the flow cytometer at the indicated times (0, 1, 3, 6 and 24 h after infection). The percentages of positive cells (M1) were indicated at each figure. |
References
1. Chomel BB, Abbott RC, Kasten RW, Floyd-Hawkins KA, Kass PH, Glaser CA, et al. Bartonella henselae prevalence in domestic cats in California: risk factors and association between bacteremia and antibody titers. J Clin Microbiol. 1995. 33:2445–2450.

2. Koehler JE, Glaser CA, Tappero JW. Rhochalimaea henselae infection. A new zoonosis with the domestic cat as reservoir. JAMA. 1994. 271:531–535.

3. Garcia-Caceres U, Garcia FU. Bartonellosis, An immunodepressive disease and the life of Daniel Alcides Carrión. Am J Clin Pathol. 1991. 95:S58–S66.
5. Ihler GM. Bartonella bacilliformis: dangerous pathogen slowly emerging from deep background. FEMS Microbiol Lett. 1996. 144:1–11.

6. Bass JW, Vincent JM, Person DA. The expanding spectrum of Bartonella infections: II. Cat-scratch disease. Pediatr infect Dis J. 1997. 16:163–179.
7. Stoler MH, Bonfiglio TA, Steigbigel RT, Pereira M. An atypical subcutaneous infection associated with acquired immune deficiency syndrome. Am J Clin Pathol. 1983. 80:714–718.

8. Slater LN, Welch DF, Hensel D, Coody DW. A newly recognized fastidious gram-negative pathogen as a cause of fever and bacteremia. N Engl J Med. 1990. 323:1587–1593.

9. Welch DF, Pickett DA, Slater LN, Steigerwalt AG, Brenner DJ. Rochalimaea henselae sp. nov., a cause of septicemia, bacillary angiomatosis, and parenchymal bacillary peliosis. J Clin Microbiol. 1992. 30:275–280.

10. Birtles RJ, Harrison TG, Saunders NA, Molyneux DH. Proposals to unify the genera Grahamella and Bartonella, with descriptions of Bartonella talpae comb. nov., Bartonella peromysci comb. nov., and three new species, Bartonella grahamii sp. nov., Bartonella taylorii sp. nov., and Bartonella doshiae sp. nov. Int J Syst Bacteriol. 1995. 45:1–8.

11. Tsukahara M, Tsuneoka H, Iino H, Ohno K, Murano I. Bartonella henselae infection from a dog. Lancet. 1998. 352:1682.
12. Bass JW, Vincent JM, Person DA. The expanding spectrum of Bartonella infections: I. Bartonellosis and trench fever. Pediatr Infect Dis J. 1997. 16:2–10.

13. Jackson LA, Perkins BA, Wenger JD. Cat scratch disease in the United States: an analysis of three national databases. Am J Public Health. 1993. 83:1707–1711.

14. Carithers HA. Cat-scratch disease. An overview based on a study of 1,200 patients. Am J Dis Child. 1985. 139:1124–1133.
15. Maurin M, Raoult D. Bartonella infections: Diagnostic and management issues. Curr Opin Infect Dis. 1998. 11:189–193.
16. Finkelstein JL, Brown TP, O'Reilly KL, Wedincamp J Jr, Foil LD. Studies on the growth of Bartonella henselae in the cat flea (Siphonaptera: Pulicidae). J Med Entomol. 2002. 39:915–919.

17. Agan BK, Dolan MJ. Laboratory diagnosis of Bartonella infections. Clin Lab Med. 2002. 22:937–962.
18. Chung JY, Koo JW, Kim SW, Yoo YS, Han TH, Lim SJ. A case of cat scratch disease confirmed by polymerase chain reaction for Bartonella henselae DNA. Korean J Pediatr. 2005. 48:789–792.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download