Abstract
Adeno-associated virus (AAV) and human papillomavirus (HPV) DNAs were found in abnormal quality semen, early abortus and female genital tissues. It was suggested that they might cause male infertility and miscarriages. This study was performed to determine the detection rate of these viruses in the semen and to assess the relationship between the presence of virus and male factor infertility and recurrent miscarriages. Sixty-three of 99 recruited male were included in this study according to the completeness of follow-up and the sample availability. Fourteen male with normal reproductive capacity were allocated to control group, 15 male with abnormal results in semen analysis were grouped as male factor infertility (MF) group, and 34 male whose spouses have had history of repeated spontaneous abortions were designated as repeated miscarriage (RM) group. AAV and HPV were detected in semen by polymerase chain reaction. The detection rate of AAV in the MF infertility group and RM group was 60.0% and 50.0%, respectively, while 14.3% in the control group (p < 0.05). However, the differences in the detection rate of HPV were not statistically significant among groups. These results suggest that AAV could be related to repeated miscarriages and male infertility.
The replication of adeno-associated virus (AAV), which is a small non-enveloped single stranded DNA virus, usually requires co-infection with helper virus (1). Among the 12 AAV serotype, AAV-2, 3, and 5 have been detected in human clinical samples (2, 3). They belong to the genus Dependovirus, family Parvoviridae (4).
Although AAV infection is yet thought to be nonpathogenic in human, there has been possibility of its association with early miscarriage and other reproductive problems during pregnancy (5~7). AAV DNA has been detected in uterus, early abortus, and female genital tissues (6, 8, 9). Its detection rate (12~77%) depends on the experimental methods and the sampling conditions. Reports on the detection rate of AAV in cervical secretion and prevalence of serum antibodies to AAV indicated that AAV infections were significantly more frequent in pregnant women than in non-pregnant controls (10, 11). AAV infection occurred frequently in male reproductive tract and viral DNA of AAV was mainly detected in sperm fractions of the semen. These findings indicate the possibility of AAV being sexually transmitted. In the previous studies, AAV was frequently detected in the semen from men with infertility (12, 13), which suggests that AAV might be a possible causative agent of male infertility. The presence of AAV in early abortus also indicates that AAV may be one of the causes of abortion (5, 7, 9). Additionally, HPV, which is a helper virus of AAV, is involved in male factor infertility and can reduce the motility of sperm (14, 15).
To assess the relationship between the presence of these viruses and male factor infertility and repeated miscarriages of their partners, the detection rates of AAV and HPV were evaluated in the semen.
From May 2007 to February 2008, the informed consent was obtained from 99 male who visited the Hamchoon Women's Clinic for sperm donation, semen analysis or in vitro fertilization and embryo transfer (IVF-ET) programs. This study was approved by Institutional Review Board in both Seoul National University Hospital and Hamchoon Women's Clinic. Semen samples were collected by masturbation following three- or four-days' sexual abstinence. Thirty-six male were excluded from study; in 4 cases, there were no remaining samples or DNA after nucleic acid extraction procedure; in 32 cases, reproductivity could not be evaluated because they did not revisit the clinic or did not report whether pregnancy was maintained successfully or not.
The semen samples were examined by routine analysis according to WHO guidelines. Abnormal semen was evaluated according to sperm concentration (less than 20 × 106/ml) or sperm motility (less than 50%). Abnormal results were shown in 15 men and they were allocated to the male factor (MF) infertility group. Thirty-four men, whose wives had suffered from more than two miscarriages, were designated as the repeated miscarriages (RM) group. We defined these two groups as an impaired reproductivity group. Fourteen men were allocated to control group; they had normal results in semen analysis and their conception partner had ongoing pregnancy to mid-trimester without any treatment for miscarriages.
DNA extraction was performed using Real Genomics™ Genomic DNA Extraction Kit (Real Biotech Corporation, Taiwan) according to the manufacturer's protocol. Achondroplasia gene amplification was used as a control of the extraction and DNA integrity (Fig. 1A).
All samples were examined for the presence of AAV by the nested PCR using AUS1/Pan3 primers (AUS1: 5'-ACA CCA TCT GGC TGT TTG GG-3', Pan3: AAA AAG TCT TTG ACT TCC TGC TT-3') for primary PCR and IN1/IN2 primers (IN1: GAG GCC ATA GCC CAC ACT GT-3', IN2: GAG AAT GGC TTT GGC CGA CT-3') for nested PCR (16). Two µL of extracted DNA of each sample was initially mixed with an AccuPower™ PCR PreMix (Bioneer, Korea) which contained 1.5 mM MgCl2, 250 µM of each dNTP, 10 mM Tris-HCl pH 9.0, 30 mM KCl, and 1.0 unit Taq polymerase. To this mixture, 20 pmole of each primer was added and adjusted to a final volume of 20 µl with deionized water. The same reaction formula was applied for nested PCR using 2 µl of tenfold-diluted primary PCR product as template. The primary PCR was carried out as follows: initial denaturation at 94℃ for 10 min, followed by 25 cycles for 30 sec at 94℃, 20 sec at 58℃, 10 sec at 72℃ and with a final extension at 72℃ for 7 min. The nested PCR reactions were carried out with an initial denaturation step at 94℃ for 10 min, 25 cycles of 30 sec at 94℃, 15 sec at 61℃ and 10 sec at 72℃ and with a final extension at 72℃ for 7 min. A volume of 5 µl products was subjected to electrophoresis on 2% agarose gel and visualized under UV illuminator with ethidium bromide staining. The primary and nested PCR resulted in 427 and 150 bp products, respectively (Fig. 1B and 1C). AAV2 strain (ATCC VR680) was used as a positive control. DNA and RNA free water was included in all PCR procedures as a negative control.
HPV DNA detection was based on PCR amplification of the L1 region using the L1A/L1B primers (L1A: 5'-GCA CAG GGA CAT AAT AAT GG-3', L1B: 5'-CGT CCA AGA GGA TAC TGA TC-3') (17). The reaction mixture was same as that of AAV detection. Initial denaturation step was at 94℃ for 10 min, followed by 35 cycles for 30 sec at 94℃, 30 sec at 60℃, and 15 sec at 72℃, and with a final extension at 72℃ for 7 min. PCR product was a 452 bp fragment.
PASW Statistics 17.0 program was used to analyze the data. Differences in the means of the continuous interval were analyzed using one-way ANOVA. Frequencies of virus DNA detection were analyzed using χ2-test; odds ratio and 95% confidence interval were calculated where appropriate. In all analyses, the value of p < 0.05 was considered significant.
To access the relationship between the detection of AAV and HPV in the semen and male infertility and recurrent miscarriages, semen analysis and amplification of specific sequences of both viruses were done.
Table 1 demonstrates differences in the sperm count and the sperm motility of three tested groups: the control, MF infertility group, and RM group. Both the sperm count (25.9 ± 27.52 × 106/ml) and the sperm motility (33.7 ± 13.95%) in MF infertility group were significantly lower than in control (62.1 ± 24.24 × 106/ml and 67.5 ± 9.15%, respectively) and RM group (72.4 ± 23.75 × 106/ml and 67.9 ± 9.62%, respectively).
Cellular DNA was detected in all semen samples (achondroplasia sequence in chromosome 4p). AAV DNA in the semen was found in 50% (17/34) of RM and 60% (9/15) of MF infertility group, which was statistically significantly higher than control group (14%, 2/14) (Table 2). Odds ratio of the MF infertility group in AAV detection compared with the control group was 9.0 (95% CI 1.46~55.48) and that compared with the RM group was 6.0 (95% CI 1.16~30.96).
HVP DNA was detected in the semen. Table 2 shows that the detection rates of HPV in RM (15%, 5/34) and MF infertility group (7%, 1/15) were not statistically different from that of control group (21%, 3/14). The co-infections of AAV with HPV were 14, 12 and 0% in the control, RM and MF infertility group, respectively.
AAV has been investigated with the purpose of carrying out gene therapy and does not seem to be pathogenic in human. However, AAV has been found to infect the genital system of both man and woman and might be associated with early miscarriages and other prenatal problems (5, 7, 9, 12, 18).
Since normal delivery can be expected if the pregnancy is maintained through the second trimester, men whose spousal pregnancies were ongoing to mid-trimester without any treatment of repeated miscarriage were used as the control group.
AAV detection rate of MF infertility group was significantly higher than control group. MF infertility group was subdivided by their semen characteristics and compared for AAV detection rate with control group. AAV positive rates in oligozoospermia, asthenozoospermia, and oligoasthenozoospermia subgroups were 67%, 43% and 80%, respectively. Differences of AAV positive rate among these subgroups and control group were statistically significant (p < 0.05), but odd ratio (24.0, 95% CI 1.69~340.99) was statistically significant only in oligoasthenozoospermia subgroup when compared with control group. AAV DNA was detected in 6 of 8 patients with abnormal sperm count (OR 18.0, 95% CI 2.01~161.04), and in 7 of 12 patients with abnormal motility (OR 8.4, 95% CI 1.27~55.39). Higher detection rate of AAV in the semen with abnormal sperm parameters implies that there is a relationship between AAV infection and male factor infertility. Although the effect of AAV on the male factor infertility has not been clearly defined, AAV DNA could be detected in the sperm and the testis of azoospermia patients (13). This report suggests that AAV may exert the harmful effect on the sperm maturation.
Based on the report that semen containing lots of large sperms could cause repeated miscarriages because of the production of chromosomally abnormal embryos (19), sperm morphology test were fulfilled among patients. AAV DNA was present in 50% of RM group, which was significantly higher than that in the control group. The report showed that AAV infection was associated with adverse reproductive outcome and that AAV DNA was found in 73% of early abortus. It suggests that there could be a relationship between repeated miscarriages and AAV infection, although AAV DNA has not been surveyed in repeated miscarriages cases (7, 9). The miscarriages might have resulted from AAV infected sperms transmitting the virus to the egg. The ability of AAV to dysregulate the cell cycle might have a deleterious effect on embryo development. AAV infection induced the cessation of cleavage at 2-cell stage and injecting AAV DNA to the fertilized mouse embryos led to the developmental blockage at morula stage. Also the fetal death and early abortion were observed in AAV-infected pregnant mice (20).
Other study demonstrated that seropositivity of IgM antibody against AAV virus in maternal serum in the first trimester was 5.6 times more prevalent in pre-eclampsia, intrauterine growth retardation, and stillbirth cases, and 7.6 times more prevalent in pre-term deliveries than in control group (18). The higher detection of IgG antibody against AAV in the serum and AAV DNA in the cervical samples of the pregnant women than in non pregnant women might result from the reactivation of AAV persisting in woman's reproductive organ. This reactivation might be due to hormonal influences during pregnancy (10, 11). In general, patients with repeated miscarriages may be pregnant for much longer period of time and more frequently than normal pregnant women with regard to the total period of pregnancy. Therefore they have more chance to transmit AAV to their conception partners. This is one of the other possible explanations for higher detection rate of AAV DNA in the semen from RM group than that from the control group. This detection possibility, however, has limitation because only the semen was tested in our study. Therefore further research is warranted to look into this possibility in women in the future.
The detection rate of AAV DNA in our study was higher than the previous reports (12, 13). The differences in the prevalence of AAV infection due to racial or geographical disparity might have had influence on the result. Detection rate of AAV DNA in healthy women was 50% in Arkansas, but that in Jamaica was 0% even though same PCR protocol and primers were used (21, 22).
A recent report by Schlehofer et al showed no significant association of the presence of AAV DNA in semen samples or endocervical swabs with clinically relevant infertility factors (23). Their results were different from ours, but profiles of recruited patients and kinds of tested samples were also different from each other. Their patients were subfertile and especially over 80 percentage of patients were male factor infertility with reduced sperm motility. But the positive rate of AAV DNA in semen was lower than this study and their previous studies (12, 13). They did not include normal males, and did not explain the reasons of lower detection rates. Some additional future studies with sufficient number of well-characterized patients and normal persons are needed.
HPV, one of AAV helper viruses, is associated with male infertility. Sperm motility was decreased by the incubation with HPV DNA E6-E7 region for 24 h (15). However, other investigators reported that the detection of HPV DNA in semen was not related with the sperm quality, i.e. sperm counts and motility (24). The result of the detection of HPV DNA in the semen did not show significant correlation between the male infertility and HPV infection in our study. In several other reports, the prevalence of HPV DNA ranged from 4.5 to 36.5% in the semen of asymptomatic men (12, 13, 24, 25, 26). The finding that the prevalence of HPV DNA in the men (14.3%, 9/63) was lower than that of meta-analysis result (23.9%) in Korean women with normal cytology (27) is in line with the result from previous reports (28, 29).
The present findings suggest that AAV could be related to repeated miscarriages and male infertility. It will be worth the effort to learn the mechanism on how AAV affects to spermatogenesis and to confirm whether AAV and helper virus decrease the success rate of IVF-ET. Additionally, the further research with women should be included to better understand the relationship between repeated miscarriages and AAV infection.
Figures and Tables
Figure 1
Gel electrophoresis analysis of PCR products from positive controls. M) 100 bp DNA ladder size marker, (A) Achondroplasia (179 bp), (B) primary PCR for AAV2 (AUS1-Pan3 427 bp), (C) nested PCR for AAV2 (IN1-IN2 150 bp), (D) HPV16 (~450 bp), (E) HPV18 (~450 bp). Electrophoresis was performed on a 2% agarose gel contained EtBr, and DNA bands were visualized under UV light.
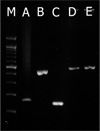
Table 2
Viral DNA detection in the semen.

aData are expressed as No. viral DNA-positive semen/No. tested sample (%)
bData are expressed as odds ratio [95% confidence interval] for AAV positive/negative between control and each group
cCo-infection means that both virus were detected in the same sample
- : Odds ratios were not calculated because detection of HPV was not significant among three groups
NS : Not significant
References
1. Malhomme O, Dutheil N, Rabreau M, Armbruster-Moraes E, Schlehofer JR, Dupressoir T. Human genital tissues containing DNA of adeno-associated virus lack DNA sequences of the helper viruses adenovirus, herpes simplex virus or cytomegalovirus but frequently contain human papillomavirus DNA. J Gen Virol. 1997. 78:1957.

2. Schlehofer JR. Virus infections in disorders of the male reproductive system. Andrologia. 2003. 35:157–159.

3. Schmidt M, Voutetakis A, Afione S, Zheng C, Mandikian D, Chiorini JA. Adeno-associated virus type 12 (AAV12): a novel AAV serotype with sialic acid- and heparan sulfate proteoglycan-independent transduction activity. J Virol. 2008. 82:1399–1406.

4. Brown KE. Zuckerman AJ, Banatvals JE, Pattison JR, Griffiths PD, Schoub BD, editors. Human Parvoviruses. Principles and practice of clinical virology. 2004. 5th ed. John Wiley & Sons Ltd;713–720.

5. Tobiasch E, Rabreau M, Geletneky K, Laruë-Charlus S, Severin F, Becker N, et al. Detection of adeno-associated virus DNA in human genital tissue and in material from spontaneous abortion. J Med Virol. 1994. 44:215–222.

6. Burguete T, Rabreau M, Fontanges-Darriet M, Roset E, Hager HD, Köppel A, et al. Evidence for infection of the human embryo with adeno-associated virus in pregnancy. Hum Reprod. 1999. 14:2396–2401.

7. Pereira CC, de Freitas LB, de Vargas PR, de Azevedo ML, do Nascimento JP, Spano LC. Molecular detection of Adeno-Associated Virus in cases of spontaneous and intentional human abortion. J Med Virol. 2010. 82:1689–1693.

8. Venturoli S, Cricca M, Bonvicini F, Gallinella G, Gentilomi G, Zerbini M, et al. Detection of adeno-associated virus DNA in female genital samples by PCR-ELISA. J Med Virol. 2001. 64:577–582.

9. Kiehl K, Schlehofer JR, Schultz R, Zugaib M, Armbruster-Moraes E. Adeno-associated virus DNA in human gestational trophoblastic disease. Placenta. 2002. 23:410–415.

10. Erles K, Sebökovà P, Schlehofer JR. Update on the prevalence of serum antibodies (IgG and IgM) to adeno-associated virus (AAV). J Med Virol. 1999. 59:406–411.

11. Freitas LB, Pereira CC, Checon R, Leite JP, Nascimento JP, Spano LC. Adeno-associated virus and human papillomavirus types in cervical samples of pregnant and non-pregnant women. Eur J Obstet Gynecol Reprod Biol. 2009. 145:41–44.

12. Rohde V, Erles K, Sattler HP, Derouet H, Wullich B, Schlehofer JR. Detection of adeno-associated virus in human semen: does viral infection play a role in the pathogenesis of male infertility? Fertil Steril. 1999. 72:814–816.

13. Erles K, Rohde V, Thaele M, Roth S, Edler L, Schlehofer JR. DNA of adeno-associated virus (AAV) in testicular tissue and in abnormal semen samples. Hum Reprod. 2001. 16:2333–2337.

14. Lai YM, Lee JF, Huang HY, Soong YK, Yang FP, Pao CC. The effect of human papillomavirus infection on sperm cell motility. Fertil Steril. 1997. 67:1152–1155.

15. Lee CA, Huang CT, King A, Chan PJ. Differential effects of human papillomavirus DNA types on p53 tumor-suppressor gene apoptosis in sperm. Gynecol Oncol. 2002. 85:511–516.

16. Zheng BY, Li XD, Wiklund F, Chowdhry S, Angstrom T, Hallmans G, et al. Detection of adeno-associated virus type2 genome in cervical carcinoma. Br J Cancer. 2006. 94:1913–1917.

17. Zheng PS, Li SR, Iwasaka T, Song J, Cui MH, Sugimori H. Simultaneous detection by consensus multiplex PCR of high- and low-risk and other types of human papilloma virus in clinical samples. Gynecol Oncol. 1995. 58:179–183.

18. Arechavaleta-Velasco F, Gomez L, Ma Y, Zhao J, McGrath CM, Sammel MD, et al. Adverse reproductive outcomes in urban women with adeno-associated virus-2 infections in early pregnancy. Hum Reprod. 2008. 23:29–36.

19. Viville S, Mollard R, Bach ML, Falquet C, Gerlinger P, Warter S. Do morphological anomalies reflect chromosomal aneuploidies?: case report. Hum Reprod. 2000. 15:2563–2566.

20. Botquin V, Cid-Arregui A, Schlehofer JR. Adeno-associated virus type 2 interferes with early development of mouse embryos. J Gen Virol. 1994. 75:2655–2662.

21. Han L, Parmley TH, Keith S, Kozlowski KJ, Smith LJ, Hermonat PL. High prevalence of adeno-associated virus (AAV) type 2 rep DNA in cervical materials: AAV may be sexually transmitted. Virus Genes. 1996. 12:47–52.

22. Strickler HD, Viscidi R, Escoffery C, Rattray C, Kotloff KL, Goldberg J, et al. Adeno-associated virus and development of cervical neoplasia. J Med Virol. 1999. 59:60–65.

23. Schlehofer JR, Boeke C, Reuland M, Eggert-Kruse W. Presence of DNA of adeno-associated virus in subfertile couples, but no association with fertility factors. Hum Reprod. 2012. 27:770–778.

24. Rintala MA, Grénman SE, Pöllänen PP, Suominen JJ, Syrjänen SM. Detection of high-risk HPV DNA in semen and its association with the quality of semen. Int J STD AIDS. 2004. 15:740–743.

25. Kim SH, Kim EG, Ku SY, Jee BC, Seo CS, Choi YM, et al. Effect of type 16 human papillomavirus positivity in uterine cervix and follicular fluid of infertile women and sperm of their spouses on outcomes of in vitro fertilization and embryo transfer. Korean J Obstet Gynecol. 2000. 43:1414–1421.
26. Bezold G, Politch JA, Kiviat NB, Kuypers JM, Wolff H, Anderson DJ. Prevalence of sexually transmissible pathogens in semen from asymptomatic male infertility patients with and without leukocytospermia. Fertil Steril. 2007. 87:1087–1097.

27. Bae JH, Lee SJ, Kim CJ, Hur SY, Park YG, Lee WC, et al. Human papillomavirus (HPV) type distribution in Korean women: a meta-analysis. J Microbiol Biotechnol. 2008. 18:788–794.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download