Abstract
In annual epidemics and occasional pandemics, influenza viruses cause acute respiratory illnesses in infected humans. Vaccines and antiviral drugs are two main arsenals available for a fight against influenza viruses. However, vaccines often exhibit a limited efficacy in high risk populations, and antiviral drugs are always concerned for mutations, which confer viral resistance. Here we review current advances and knowledge in relation to the usage of antiviral drugs as a prophylactic or therapeutic and the mechanism of resistant variants mainly against the neuraminidase inhibitors. Comprehensive understanding of the resistant mechanism will pave a road for developing new antivirals and/or finding medical or natural alternatives inducing less frequent resistance, and application of combination therapy using two or three different kinds of antivirals can suggest a useful medical intervention against both of seasonal and highly pathogenic influenza viruses including resistant variants. In this review, we provide insights of antiviral drugs for the control and prevention of influenza viruses.
Figures and Tables
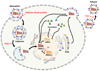 | Figure 1
Antiviral agents against influenza virus. Sialidase fusion protein blocks the binding of influenza virus to its cellular receptors in the cell surface. M2 ion channel inhibitors (M2I) block the ion channel produced by M2 protein. Neuraminidase inhibitors (NAI) block the process of release of influenza viruses from infected cells. Polymerase inhibitor (PI) inhibits influenza virus polymerase activity. |
Table 1
Summary of NA mutations conferring resistance to neuraminidase inhibitors.

aN2 numbering
bS, susceptible; I, intermediate (5-fold < wild-type IC50 < 10-fold); R, resistant (>10-fold increase in wild-type IC50); based on the NAI susceptibility definition of Neuraminidase Inhibitor Surveillance Network (www.nisn.org)
References
1. WHO. Fact sheet: influenza (seasonal). 2009. www.who.int/mediacentre/factsheets/fs211/en/.
2. Palese P, Shaw ML. Fileds Virology, Ch.47 Orthomyxoviridae: The viruses and their replication. 2007. 5th ed. Lippincott Williams & Wilkins.
3. Wright PF, Neumann G, Kawaoka Y. Fileds Virology, Ch.48 Orthomyxoviruses. 2007. 5th ed. Lippincott Williams & Wilkins.
4. Clark NM, Lynch JP 3rd. Influenza: epidemiology, clinical features, therapy, and prevention. Semin Respir Crit Care Med. 2011. 32:373–392.

6. Taubenberger JK, Reid AH, Janczewski TA, Fanning TG. Integrating historical, clinical and molecular genetic data in order to explain the origin and virulence of the 1918 Spanish influenza virus. Philos Trans R Soc Lond B Biol Sci. 2001. 356:1829–1839.

7. Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, et al. Antigenic and genetic characteristics of swine-origin 2009 A (H1N1) influenza viruses circulating in humans. Science. 2009. 325:197–201.
8. Maines TR, Jayaraman A, Belser JA, Wadford DA, Pappas C, Zeng H, et al. Transmission and pathogenesis of swine-origin 2009 A (H1N1) influenza viruses in ferrets and mice. Science. 2009. 325:484–487.

9. Dawood FS, Iuliano AD, Reed C, Meltzer MI, Shay DK, Cheng PY, et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis. 2012. 12:687–695.

10. Russell CA, Fonville JM, Brown AE, Burke DF, Smith DL, James SL, et al. The potential for respiratory droplet-transmissible A/H5N1 influenza virus to evolve in a mammalian host. Science. 2012. 336:1541–1547.

11. Herfst S, Schrauwen EJ, Linster M, Chutinimitkul S, de Wit E, Munster VJ, et al. Airborne transmission of influenza A/H5N1 virus between ferrets. Science. 2012. 336:1534–1541.

12. Centers for Disease Control and Prevention (CDC). Swine-origin influenza A (H3N2) virus infection in two cildren--Indiana and Pennsylvania, July-August 2011. MMWR Morb Mortal Wkly Rep. 2011. 60:1213–1215.
13. Centers for Disease Control and Prevention (CDC). Notes from the field: Highly pathogenic avian influenza A (H7N3) virus infection in two poultry workers -- Jalisco, Mexico, July 2012. MMWR Morb Mortal Wkly Rep. 2012. 61:726–727.
14. de Jong JC, Beyer WE, Palache AM, Rimmelzwaan GF, Osterhaus AD. Mismatch between the 1997/1998 influenza vaccine and the major epidemic A (H3N2) virus strain as the cause of an inadequate vaccine-induced antibody response to this strain in the elderly. J Med Virol. 2000. 61:94–99.

15. Skowronski DM, Masaro C, Kwindt TL, Mak A, Petric M, Li Y, et al. Estimating vaccine effectiveness against laboratory-confirmed influenza using a sentinel physician network: results from the 2005-2006 season of dual A and B vaccine mismatch in Canada. Vaccine. 2007. 25:2842–2851.

16. Sugaya N, Tamura D, Yamazaki M, Ichikawa M, Kawakami C, Kawaoka Y, et al. Comparison of the clinical effectiveness of oseltamivir and zanamivir against influenza virus infection in children. Clin Infect Dis. 2008. 47:339–345.

17. Carrat F, Duval X, Tubach F, Mosnier A, Van der Werf S, Tibi A, et al. Effect of oseltamivir, zanamivir or oseltamivir-zanamivir combination treatments on transmission of influenza in households. Antivir Ther. 2012. 17:1085–1090.

18. Lapinsky SE. H1N1 novel influenza A in pregnant and immunocompromised patients. Crit Care Med. 2010. 38:e52–e57.

19. Jackson RJ, Cooper KL, Tappenden P, Rees A, Simpson EL, Read RC, et al. Oseltamivir, zanamivir and amantadine in the prevention of influenza: a systematic review. J Infect. 2011. 62:14–25.

21. He G, Massarella J, Ward P. Clinical pharmacokinetics of the prodrug oseltamivir and its active metabolite Ro 64-0802. Clin Pharmacokinet. 1999. 37:471–484.

22. Davies BE. Pharmacokinetics of oseltamivir: an oral antiviral for the treatment and prophylaxis of influenza in diverse populations. J Antimicrob Chemother. 2010. 65:Suppl 2. ii5–ii10.

23. Nguyen HT, Fry AM, Gubareva LV. Neuraminidase inhibitor resistance in influenza viruses and laboratory testing methods. Antivir Ther. 2012. 17:159–173.

24. Monto AS, McKimm-Breschkin JL, Macken C, Hampson AW, Hay A, Klimov A, et al. Detection of influenza viruses resistant to neuraminidase inhibitors in global surveillance during the first 3 years of their use. Antimicrob Agents Chemother. 2006. 50:2395–2402.

25. Meijer A, Lackenby A, Hungnes O, Lina B, van-der-Werf S, Schweiger B, et al. Oseltamivir-resistant influenza virus A (H1N1), Europe, 2007-08 season. Emerg Infect Dis. 2009. 15:552–560.

26. WHO. Influenza A (H1N1) virus resistance to oseltamivir. WHO, Geneva, Switzerland. 2009. www.who.int/csr/disease/influenza/h1n1_table/en/.
27. Dharan NJ, Gubareva LV, Meyer JJ, Okomo-Adhiambo M, McClinton RC, Marshall SA, et al. Infections with oseltamivir-resistant influenza A (H1N1) virus in the United States. JAMA. 2009. 301:1034–1041.

28. Kramarz P, Monnet D, Nicoll A, Yilmaz C, Ciancio B. Use of oseltamivir in 12 European countries between 2002 and 2007--lack of association with the appearance of oseltamivir-resistant influenza A (H1N1) viruses. Euro Surveill. 2009. 14:pii: 19112.
29. Ives JA, Carr JA, Mendel DB, Tai CY, Lambkin R, Kelly L, et al. The H274Y mutation in the influenza A/H1N1 neuraminidase active site following oseltamivir phosphate treatment leave virus severely compromised both in vitro and in vivo. Antiviral Res. 2002. 55:307–317.

30. Bloom JD, Gong LI, Baltimore D. Permissive secondary mutations enable the evolution of influenza oseltamivir resistance. Science. 2010. 328:1272–1275.

31. Baz M, Abed Y, McDonald J, Boivin G. Characterization of multidrug-resistant influenza A/H3N2 viruses shed during 1 year by an immunocompromised child. Clin Infect Dis. 2006. 43:1555–1561.

32. Pizzorno A, Bouhy X, Abed Y, Boivin G. Generation and characterization of recombinant pandemic influenza A (H1N1) viruses resistant to neuraminidase inhibitors. J Infect Dis. 2011. 203:25–31.

33. Kiso M, Ozawa M, Le MT, Imai H, Takahashi K, Kakugawa S, et al. Effect of an asparagine-to-serine mutation at position 294 in neuraminidase on the pathogenicity of highly pathogenic H5N1 influenza A virus. J Virol. 2011. 85:4667–4672.

34. Mitnaul LJ, Matrosovich MN, Castrucci MR, Tuzikov AB, Bovin NV, Kobasa D, et al. Balanced hemagglutinin and neuraminidase activities are critical for efficient replication of influenza A virus. J Virol. 2000. 74:6015–6020.

35. Ginting TE, Shinya K, Kyan Y, Makino A, Matsumoto N, Kaneda S, et al. Amino acid changes in hemagglutinin contribute to the replication of oseltamivir-resistant H1N1 influenza viruses. J Virol. 2012. 86:121–127.

36. Bouvier NM, Rahmat S, Pica N. Enhanced mammalian transmissibility of seasonal influenza A/H1N1 viruses encoding an oseltamivir-resistant neuraminidase. J Virol. 2012. 86:7268–7279.

37. Malakhov MP, Aschenbrenner LM, Smee DF, Wandersee MK, Sidwell RW, Gubareva LV, et al. Sialidase fusion protein as a novel broad-spectrum inhibitor of influenza virus infection. Antimicrob Agents Chemother. 2006. 50:1470–1479.

38. Belser JA, Lu X, Szretter KJ, Jin X, Aschenbrenner LM, Lee A, et al. DAS181, a novel sialidase fusion protein, protects mice from lethal avian influenza H5N1 virus infection. J Infect Dis. 2007. 196:1493–1499.

39. Triana-Baltzer GB, Gubareva LV, Nicholls JM, Pearce MB, Mishin VP, Belser JA, et al. Novel pandemic influenza A (H1N1) viruses are potently inhibited by DAS181, a sialidase fusion protein. PLoS One. 2009. 4:e7788.
40. Hedlund M, Aschenbrenner LM, Jensen K, Larson JL, Fang F. Sialidase-based anti-influenza virus therapy protects against secondary pneumococcal infection. J Infect Dis. 2010. 201:1007–1015.

42. Brooks MJ, Sasadeusz JJ, Tannock GA. Antiviral chemotherapeutic agents against respiratory viruses: where are we now and what's in the pipeline? Curr Opin Pulm Med. 2004. 10:197–203.
43. Furuta Y, Takahashi K, Shiraki K, Sakamoto K, Smee DF, Barnard DL, et al. T-705 (favipiravir) and related compounds: Novel broad-spectrum inhibitors of RNA viral infections. Antiviral Res. 2009. 82:95–102.

44. Kohno S, Yen MY, Cheong HJ, Hirotsu N, Ishida T, Kadota J, et al. Phase III randomized, double-blind study comparing single-dose intravenous peramivir with oral oseltamivir in patients with seasonal influenza virus infection. Antimicrob Agents Chemother. 2011. 55:5267–5276.

45. Kohno S, Kida H, Mizuguchi M, Hirotsu N, Ishida T, Kadota J, et al. Intravenous peramivir for treatment of influenza A and B virus infection in high-risk patients. Antimicrob Agents Chemother. 2011. 55:2803–2812.

46. Birnkrant D, Cox E. The Emergency Use Authorization of peramivir for treatment of 2009 H1N1 influenza. N Engl J Med. 2009. 361:2204–2207.

47. Ikematsu H, Kawai N. Laninamivir octanoate: a new long-acting neuraminidase inhibitor for the treatment of influenza. Expert Rev Anti Infect Ther. 2011. 9:851–857.

48. Yuen KY, Chan PK, Peiris M, Tsang DN, Que TL, Shortridge KF, et al. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet. 1998. 351:467–471.

49. de Jong MD, Simmons CP, Thanh TT, Hien VM, Smith GJ, Chau TN, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006. 12:1203–1207.

50. Salomon R, Hoffmann E, Webster RG. Inhibition of the cytokine response does not protect against lethal H5N1 influenza infection. Proc Natl Acad Sci U S A. 2007. 104:12479–12481.

51. Beigel JH, Farrar J, Han AM, Hayden FG, Hyer R, de Jong MD, et al. Avian influenza A (H5N1) infection in humans. N Engl J Med. 2005. 353:1374–1385.

52. Chotpitayasunondh T, Ungchusak K, Hanshaoworakul W, Chunsuthiwat S, Sawanpanyalert P, Kijphati R, et al. Human disease from influenza A (H5N1), Thailand, 2004. Emerg Infect Dis. 2005. 11:201–209.

53. Darwish I, Mubareka S, Liles WC. Immunomodulatory therapy for severe influenza. Expert Rev Anti Infect Ther. 2011. 9:807–822.

54. Smee DF, Bailey KW, Morrison AC, Sidwell RW. Combination treatment of influenza A virus infections in cell culture and in mice with the cyclopentane neuraminidase inhibitor RWJ-270201 and ribavirin. Chemotherapy. 2002. 48:88–93.

55. Smee DF, Hurst BL, Wong MH, Bailey KW, Tarbet EB, Morrey JD, et al. Effects of the combination of favipiravir (T-705) and oseltamivir on influenza A virus infections in mice. Antimicrob Agents Chemother. 2010. 54:126–133.

56. Smee DF, Hurst BL, Wong MH, Tarbet EB, Babu YS, Klumpp K, et al. Combinations of oseltamivir and peramivir for the treatment of influenza A (H1N1) virus infections in cell culture and in mice. Antiviral Res. 2010. 88:38–44.

57. Masihi KN, Schweiger B, Finsterbusch T, Hengel H. Low dose oral combination chemoprophylaxis with oseltamivir and amantadine for influenza A virus infections in mice. J Chemother. 2007. 19:295–303.

58. Tarbet EB, Maekawa M, Furuta Y, Babu YS, Morrey JD, Smee DF. Combinations of favipiravir and peramivir for the treatment of pandemic influenza A/California/04/2009 (H1N1) virus infections in mice. Antiviral Res. 2012. 94:103–110.

59. Galabov AS, Simeonova L, Gegova G. Rimantadine and oseltamivir demonstrate synergistic combination effect in an experimental infection with type A (H3N2) influenza virus in mice. Antivir Chem Chemother. 2006. 17:251–258.

60. Bantia S, Kellogg D, Parker CD, Babu YS. Combination of peramivir and rimantadine demonstrate synergistic antiviral effects in sub-lethal influenza A (H3N2) virus mouse model. Antiviral Res. 2010. 88:276–280.

61. Smee DF, Hurst BL, Egawa H, Takahashi K, Kadota T, Furuta Y. Intracellular metabolism of favipiravir (T-705) in uninfected and influenza A (H5N1) virus-infected cells. J Antimicrob Chemother. 2009. 64:741–746.

62. Ilyushina NA, Hoffmann E, Salomon R, Webster RG, Govorkova EA. Amantadine-oseltamivir combination therapy for H5N1 influenza virus infection in mice. Antivir Ther. 2007. 12:363–370.

63. Ilyushina NA, Hay A, Yilmaz N, Boon AC, Webster RG, Govorkova EA. Oseltamivir-ribavirin combination therapy for highly pathogenic H5N1 influenza virus infection in mice. Antimicrob Agents Chemother. 2008. 52:3889–3897.

64. Smee DF, Wong MH, Bailey KW, Sidwell RW. Activities of oseltamivir and ribavirin used alone and in combination against infections in mice with recent isolates of influenza A (H1N1) and B viruses. Antivir Chem Chemother. 2006. 17:185–192.

66. Evering TH, Mehandru S, Racz P, Tenner-Racz K, Poles MA, Figueroa A, et al. Absence of HIV-1 evolution in the gut-associated lymphoid tissue from patients on combination antiviral therapy initiated during primary infection. PLoS Pathog. 2012. 8:e1002506.

67. Beigel J, Bray M. Current and future antiviral therapy of severe seasonal and avian influenza. Antiviral Res. 2008. 78:91–102.

68. Nguyen JT, Hoopes JD, Smee DF, Prichard MN, Driebe EM, Engelthaler DM, et al. Triple combination of oseltamivir, amantadine, and ribavirin displays synergistic activity against multiple influenza virus strains in vitro. Antimicrob Agents Chemother. 2009. 53:4115–4126.

69. Petersen E, Keld DB, Ellermann-Eriksen S, Gubbels S, Ilkjaer S, Jensen-Fangel S, et al. Failure of combination oral oseltamivir and inhaled zanamivir antiviral treatment in ventilator- and ECMO-treated critically ill patients with pandemic influenza A (H1N1)v. Scand J Infect Dis. 2011. 43:495–503.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download