Abstract
Viruses initiate a number of cellular stress responses and modulate gene regulation and compartmentalization of RNA upon infection to be successful parasites. Virus infections may induce or impair stress granule (SG) formation to maximize replication efficiency. SGs and processing bodies (PBs) are the RNA granules, which contain translationally inactive pool of transcripts as the mRNA silencing foci. PBs and SGs, the highly conserved macromolecular aggregates, can release mRNAs to allow their translations. Unlike constitutively existing PBs that can respond to stimuli and affect mRNA translation and decay, SGs are specifically induced upon cellular stress and can triggers a global translational silencing by several pathways, including phosphorylation of the key translation initiation factor eIF2alpha, tRNA cleavage, and sequestration of cellular components and so on. The dynamics of PBs and SGs are regulated by several signaling pathways, including histone deacetylase 6, and depend on microfilaments and microtubules, and the cognate molecular motors myosin, dynein, and kinesin. SGs share features with aggresomes and related aggregates of unfolded proteins and may play a role in the pathology. The recent advances in understanding the relationship between viruses and mRNA stress granules are summarized.
Figures and Tables
Figure 1
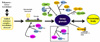
Several viruses inhibit stress granule (SG) formations (modified from White and Lloyd. Ref. 1). Sequestrations or cleavage of SG components are illustrated among several mechanisms. The inhibition of SG formations by the interaction of human T-cell leukemia virus type 1 (HTLV-1) Tax protein and histone deacetylase 6 (HDAC6) (dotted box) is emphasized to demonstrate that HDAC6 is required to localize SG by motor proteins associated microtubule transport. Deacetylase activity of HDAC6 may also function in SG assembly.
References
1. White JP, Lloyd RE. Regulation of stress granules in virus systems. Trends Microbiol. 2012. 20:175–183.

2. Thomas MG, Loschi M, Desbats MA, Boccaccio GL. RNA granules: the good, the bad and the ugly. Cell Signal. 2011. 23:324–334.

3. Kwon S, Zhang Y, Matthias P. The deacetylase HDAC6 is a novel critical component of stress granules involved in the stress response. Genes Dev. 2007. 21:3381–3394.

4. Qin Q, Hastings C, Miller CL. Mammalian orthoreovirus particles induce and are recruited into stress granules at early times postinfection. J Virol. 2009. 83:11090–11101.

5. Qin Q, Carroll K, Hastings C, Miller CL. Mammalian orthoreovirus escape from host translational shutoff correlates with stress granule disruption and is independent of eIF2alpha phosphorylation and PKR. J Virol. 2011. 85:8798–8810.

6. Smith JA, Schmechel SC, Raghavan A, Abelson M, Reilly C, Katze MG, et al. Reovirus induces and benefits from an integrated cellular stress response. J Virol. 2006. 80:2019–2033.

7. McInerney GM, Kedersha NL, Kaufman RJ, Anderson P, Liljeström P. Importance of eIF2alpha phosphorylation and stress granule assembly in alphavirus translation regulation. Mol Biol Cell. 2005. 16:3753–3763.

8. White JP, Cardenas AM, Marissen WE, Lloyd RE. Inhibition of cytoplasmic mRNA stress granule formation by a viral proteinase. Cell Host Microbe. 2007. 2:295–305.

9. Piotrowska J, Hansen SJ, Park N, Jamka K, Sarnow P, Gustin KE. Stable formation of compositionally unique stress granules in virus-infected cells. J Virol. 2010. 84:3654–3665.

10. Montero H, Rojas M, Arias CF, López S. Rotavirus infection induces the phosphorylation of eIF2alpha but prevents the formation of stress granules. J Virol. 2008. 82:1496–1504.

11. Emara MM, Brinton MA. Interaction of TIA-1/TIAR with West Nile and dengue virus products in infected cells interferes with stress granule formation and processing body assembly. Proc Natl Acad Sci U S A. 2007. 104:9041–9046.

12. Abrahamyan LG, Chatel-Chaix L, Ajamian L, Milev MP, Monette A, Clément JF, et al. Novel Staufen1 ribonucleoproteins prevent formation of stress granules but favour encapsidation of HIV-1 genomic RNA. J Cell Sci. 2010. 123:369–383.

13. Legros S, Boxus M, Gatot JS, Van Lint C, Kruys V, Kettmann R, et al. The HTLV-1 Tax protein inhibits formation of stress granules by interacting with histone deacetylase 6. Oncogene. 2011. 30:4050–4062.

14. Esclatine A, Taddeo B, Roizman B. The UL41 protein of herpes simplex virus mediates selective stabilization or degradation of cellular mRNAs. Proc Natl Acad Sci U S A. 2004. 101:18165–18170.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download