Abstract
The endoplasmic reticulum (ER) plays a crucial role in various cellular activities and cell survival. Almost all of the resident proteins usually enter the ER, and are modified with N-linked glycans and folded into the appropriate secondary and tertiary structures. When cells are faced with stressful conditions, unfolded proteins are accumulated in the ER. The discrepancies between the protein folding capacities and client protein load lead to ER stress. If the stress is prolonged, ER stress responses can activate apoptosis. ER stress-mediated apoptosis is implicated in the pathophysiology of human diseases, including several neurodegenerative diseases, diabetes mellitus, and various infectious diseases. Thus, the ER is now considered as an important organelle that can decide cell survival or death. In this review, the recent progress on ER stress and apoptosis is summarized.
Figures and Tables
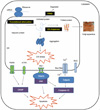 | Figure 1
ER stress response and apoptosis. On ER stress, IRE1, PERK, and ATF6 proteins initiate signal transduction that controls cell survival or death. There are at least three different mechanisms to induce ER stress; translational attenuation to avoid further accumulation of misfolded ER proteins; transcriptional activation of genes encoding ER-resident molecular chaperones; and the ER-associated degradation (ERAD) pathway to restore the folding capacity. If the cells are exposed to prolonged and strong ER stress, the damaged cells are destroyed by apoptosis. |
Notes
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0008352), and by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. 2011-0027459).
References
1. Lin JH, Walter P, Yen TS. Endoplasmic reticulum stress in disease pathogenesis. Annu Rev Pathol. 2008. 3:399–425.

2. Oyadomari S, Araki E, Mori M. Endoplasmic reticulum stress-mediated apoptosis in pancreatic beta-cells. Apoptosis. 2002. 7:335–345.
3. Schröder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005. 74:739–789.

5. Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999. 13:1211–1233.

6. Boyce M, Yuan J. Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell Death Differ. 2006. 13:363–373.

7. Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002. 415:92–96.

8. Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell. 1999. 10:3787–3799.

9. Ye J, Rawson RB, Komuro R, Chen X, Dave UP, Prywes R, et al. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell. 2000. 6:1355–1364.

10. Mori K. Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell. 2000. 101:451–454.

11. Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004. 11:381–389.

13. Gotoh T, Terada K, Oyadomari S, Mori M. hsp70-DnaJ chaperone pair prevents nitric oxide- and CHOP-induced apoptosis by inhibiting translocation of Bax to mitochondria. Cell Death Differ. 2004. 11:390–402.

14. Brush MH, Weiser DC, Shenolikar S. Growth arrest and DNA damage-inducible protein GADD34 targets protein phosphatase 1 alpha to the endoplasmic reticulum and promotes dephosphorylation of the alpha subunit of eukaryotic translation initiation factor 2. Mol Cell Biol. 2003. 23:1292–1303.

15. Du K, Herzig S, Kulkarni RN, Montminy M. TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science. 2003. 300:1574–1577.

16. Endo M, Mori M, Akira S, Gotoh T. C/EBP homologous protein (CHOP) is crucial for the induction of caspase-11 and the pathogenesis of lipopolysaccharide-induced inflammation. J Immunol. 2006. 176:6245–6253.

17. Choi HH, Shin DM, Kang G, Kim KH, Park JB, Hur GM, et al. Endoplasmic reticulum stress response is involved in Mycobacterium tuberculosis protein ESAT-6-mediated apoptosis. FEBS Lett. 2010. 584:2445–2454.

18. Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000. 403:98–103.

19. Nakagawa T, Yuan J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol. 2000. 150:887–894.
20. Hitomi J, Katayama T, Eguchi Y, Kudo T, Taniguchi M, Koyama Y, et al. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Abeta-induced cell death. J Cell Biol. 2004. 165:347–356.

21. Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006. 7:880–885.

22. Annis MG, Yethon JA, Leber B, Andrews DW. There is more to life and death than mitochondria: Bcl-2 proteins at the endoplasmic reticulum. Biochim Biophys Acta. 2004. 1644:115–123.

23. Klee M, Pimentel-Muiños FX. Bcl-X(L) specifically activates Bak to induce swelling and restructuring of the endoplasmic reticulum. J Cell Biol. 2005. 168:723–734.

24. Xu C, Xu W, Palmer AE, Reed JC. BI-1 regulates endoplasmic reticulum Ca2+ homeostasis downstream of Bcl-2 family proteins. J Biol Chem. 2008. 283:11477–11484.

25. Onuki R, Bando Y, Suyama E, Katayama T, Kawasaki H, Baba T, et al. An RNA-dependent protein kinase is involved in tunicamycin-induced apoptosis and Alzheimer's disease. EMBO J. 2004. 23:959–968.

26. Su HL, Liao CL, Lin YL. Japanese encephalitis virus infection initiates endoplasmic reticulum stress and an unfolded protein response. J Virol. 2002. 76:4162–4171.

27. Tardif KD, Waris G, Siddiqui A. Hepatitis C virus, ER stress, and oxidative stress. Trends Microbiol. 2005. 13:159–163.

28. Hurtley SM, Bole DG, Hoover-Litty H, Helenius A, Copeland CS. Interactions of misfolded influenza virus hemagglutinin with binding protein (BiP). J Cell Biol. 1989. 108:2117–2126.

29. Watowich SS, Morimoto RI, Lamb RA. Flux of the paramyxovirus hemagglutinin-neuraminidase glycoprotein through the endoplasmic reticulum activates transcription of the GRP78-BiP gene. J Virol. 1991. 65:3590–3597.

30. Ng DT, Randall RE, Lamb RA. Intracellular maturation and transport of the SV5 type II glycoprotein hemagglutinin-neuraminidase: specific and transient association with GRP78-BiP in the endoplasmic reticulum and extensive internalization from the cell surface. J Cell Biol. 1989. 109:3273–3289.

31. Pillich H, Loose M, Zimmer KP, Chakraborty T. Activation of the unfolded protein response by Listeria monocytogenes. Cell Microbiol. 2012. 14:949–964.
32. Lim YJ, Choi JA, Choi HH, Cho SN, Kim HJ, Jo EK, et al. Endoplasmic reticulum stress pathway-mediated apoptosis in macrophages contributes to the survival of Mycobacterium tuberculosis. PLoS One. 2011. 6:e28531.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download