Abstract
The production of extracellular vesicles is a ubiquitous process in both Gram-negative and Gram-positive bacteria. Gram-negative bacteria produce and secrete outer membrane vesicles during in vitro culture and in vivo infection and their contribution to bacterial pathogenesis has been well characterized. However, little is known about extracellular vesicles in Gram-positive bacteria. Until now, only few Gram-positive bacterial species, Staphylococcus aureus, Bacillus anthracis, B. cereus, and B. subtilis, have been found to produce membrane vesicles (MVs), but their contribution to bacterial pathogenesis has not been understood. Here, I discuss S. aureus MVs in terms of MV production, interaction of MVs with host cells, and immune response against MVs to understand its potential role in S. aureus pathogenesis.
A variety of Gram-negative bacteria, including pathogenic and environmental bacteria, naturally secretes extracellular vesicles, so called outer membrane vesicles (OMVs) (1~9). These extracellular vesicles are 'blebbed off' from the outer membrane when a portion of the outer membrane bulges out (10, 11). OMVs are spherical and bilayered nanovesicles with a diameter of 20~300 nm. Based on the biogenesis model proposed by Kuehn and Kesty (1), OMVs reflect the outer membrane and periplasmic components and they are composed of lipopolysaccharides (LPS), phospholipids, outer membrane proteins, and periplasmic proteins (5, 12, 13). Recent proteomic analyses reveal that cytosolic and inner membrane proteins are also packaged in the OMVs (5, 14). In addition, a wide variety of virulence determinants, such as toxins, adhesins, enzymes, and LPS, and pathogen-associated molecular patterns (PAMPs) has been found in the OMVs (8, 15~18). These vesicles play a role in cell-to-cell communication, nutrient sensing, killing of competing bacteria, the delivery of toxins or other virulence determinant to host cells, and modulation of host immune response. Examination of animal infection model and human biopsy specimens provides the evidence that Gram-negative pathogens can secrete OMVs during in vivo infection (19~22). OMVs may directly or indirectly contribute to bacterial pathogenesis during infection.
The OMV production in Gram-negative bacteria has been observed for more than 50 years, but the extracellular vesicle production in Gram-positive bacteria has not been focused, because of the absence of outer membrane in Gram-positive bacteria. However, mammalian cells have been found to secrete extracellular vesicles, so called microvesicles (23~25). The extracellular vesicle production was also identified in eukaryotic pathogens such as Cryptococcus neoformans (26, 27). These findings strongly implicate that Gram-positive bacteria can also produce extracellular vesicles. The possible evidence of extracellular vesicles in Gram-positive bacteria has been reported in 1990. Using transmission electron microscopic (TEM) analysis, the extracellular vesicles were first observed in the surfaces of Bacillus cereus and B. subtilis (28). More recently, Staphylococcus aureus and B. anthracis have been found to produce extracellular vesicles, called membrane vesicles (MVs), during in vitro culture (29, 30). The formation of extracellular vesicles and their secretion into extracellular milieu appear to be a conserved process among both Gram-negative and Gram-positive bacteria, which are considered to be a widely used strategy for pathogenic bacteria to deliver a noxious cargo to host cells during infection. This review will focus on the MV production in S. aureus and the possible contribution of S. aureus MVs to bacterial pathogenesis.
S. aureus is a Gram-positive, non-spore forming bacterium which causes a wide range of diseases in humans and animals, from uncomplicated local infection to life threatening systemic infection (31~34). The ability of S. aureus to develop diseases is associated with various virulence determinants, including structural molecules (capsule and protein A), toxins (cytotoxins, exfoliative toxins, enterotoxins, and toxic shock syndrome toxin-1), and enzymes (coagulase, catalase, hyaluronidase, fibrinolysin, lipase, and nuclease) (35, 36). Although Gram-positive bacteria usually secrete proteins via the Sec pathway (37), the secretion of these virulence determinants from S. aureus has not been well understood. Lee et al. (30) for the first time provided the evidence that Gram-positive bacterium, S. aureus ATCC 14458, produced MVs using TEM analysis. Similar to OMVs in Gram-negative bacteria, MVs purified from broth culture of S. aureus are spherical, bilayered, and membranous structures. The size of S. aureus MVs ranges from 20 to 100 nm, which are relatively smaller than OMVs derived from Gram-negative bacteria. S. aureus produces 3.47 ± 1.3 of MVs per single bacterium. More recently, Gurung et al. (38) demonstrated the production and secretion of MVs in clinical isolates, S. aureus 103D (toxic-shock syndrome toxin-1-producing strain) and 06ST1048 (methicillin-resistant S. aureus, MRSA) as well as type strains, ATCC 25923 (control strain for antimicrobial susceptibility test) and ATCC 700699 (vancomycin-intermediate S. aureus Mu50). The size of MVs purified from S. aureus 06ST1048 ranges from 20 to 130 nm, which are similar to S. aureus ATCC 14458. Moreover, they first reported that S. aureus secreted MVs during in vivo infection in a mouse pneumonia model. In the infected lung tissues, MVs are budded from bacterial surface and the secreted MVs are found in the surrounding milieu of S. aureus. These findings indicate that S. aureus produces and secretes MVs during both in vitro culture and in vivo infection (Fig. 1).
Due to the structural differences in the cell walls between Gram-positive and Gram-negative bacteria, Gram-positive bacteria may have a different biogenesis mechanism of MVs, which may result in compositional differences between Gram-positive bacterial MVs and Gram-negative bacterial OMVs. With a proteomic analysis, a total of 90 proteins were identified in the MVs of S. aureus ATCC 14458 (30), whereas a total of 143 proteins were identified in the MVs of clinical isolate of S. aureus 06ST1048 (38). Differences in protein composition between MVs of two S. aureus strains are possibly due to the bacterial strains and/or analysis techniques applied, such as the MV preparation and proteome analysis tool. Cytoplasmic proteins are the most common in the S. aureus MVs, which accounts for 56.7%, followed by extracellular (23.3%) and membrane proteins (16.7%) (30). There are some differences in the predominance of MV proteins between two S. aureus strains: IgG-binding protein, serine hydroxymethyltransferase, glyceraldehyde-3-phosphate dehydrogenase 1, staphylococcal secretory antigen, and NADH dehydrogenase-like protein are the most common in the MVs of S. aureus ATCC 14458, whereas a putative pyruvate dehydrogenase E1 component β subunit and dihydrolipoamide acetyltransferase component of pyruvate dehydrogenase complex are the most common in the MVs of S. aureus 06ST1048.
Several virulence-associated proteins are also found in the S. aureus MVs. β-lactamases packaged in the S. aureus MVs inactivate nitrocefin, chromogenic cephalosporin substrate (38), suggesting the biologically active β-lactamases in the S. aureus MVs. In addition, a variety of virulence factors associated with bacterial persistence in the infected host, tissue destruction, and induction of immune response are detected in the S. aureus MVs: they include superantigens, toxins, tissue destruction enzymes, immune evading factors, and proteins associated with bacterial adherence to host cells (Table 1). Therefore, S. aureus MVs may directly or indirectly contribute to disease development and progression.
OMVs of Gram-negative bacteria are delivered to the cytoplasm of host cells via either receptor-mediated endocytic pathway or the fusion with host cell plasma membranes (2, 39), although the fusion of OMVs with plasma membrane of host cells is still under debate. Lipid rafts on the plasma membrane of host cells are essential for the delivery of OMV components to host cells (16, 40, 41). Toxins or other molecules on the vesicular membrane can bind specifically to the receptors within lipid rafts. LPS is responsible for the binding of Pseudomonas aeruginosa OMVs to macrophage surfaces (8).
Like the delivery of OMV components to host cells, MV components of S. aureus are likely to enter the cytoplasm of host cells via a cholesterol-rich membrane microdomain (38). Two documented MV proteins, protein A and β-lactamases, are detected in the cytoplasm of epithelial HEp-2 cells treated with S. aureus MVs. Protein A is first detected in the cytoplasm of epithelial HEp-2 cells within 5 min and then persist for 24 h after treatment (unpublished data). However, MV proteins are not detected in the cells when cells are pretreated with a cholesterol destructing agent, methyl-β-cyclodextrin (MβCD). Moreover, MV components are rarely detected in the cytoplasm of host cells when cells are treated with MVs lysed with ethylenediaminetetraacetic acid (EDTA) (38). These findings suggest that MVs are an important vehicle for the delivery of bacterial components, including virulence determinants, to host cells. However, future studies will clearly be necessary to identify other delivery mechanisms of MVs and determine the bacterial molecules associated with lipid rafts. The subsequent trafficking pathway of MVs following the entry should be also clarified.
OMVs derived from several pathogenic Gram-negative bacteria, including Acinetobacter baumannii, enterotoxigenic Escherichia coli, and P. aeruginosa, induce cytotoxicity of host cells (8, 16, 41). B. anthracis MVs containing toxin components, such as protective antigen, lethal factor, and edema factor, can also induce cytotoxicity of macrophages (29). It has been recently reported that MVs of clinical MRSA 06ST1048 isolate induce morphological changes of HEp-2 cells, such as cellular shrinkage, nuclear condensation, and nuclear fragmentation, and then finally result in apoptotic cell death (38). The cytotoxicity of S. aureus MVs on epithelial HEp-2 cells is apparently in dose- and time-dependent manners. However, neither MVs lysed with EDTA nor pretreatment of MβCD in the cells induces cytotoxicity of epithelial cells. To clarify whether cytotoxicity is a common phenomenon in S. aureus MVs, MVs were purified from different S. aureus strains and treated to HEp-2 cells. However, no cytotoxicity was observed in HEp-2 cells treated with MVs from other S. aureus strains, except from S. aureus 06ST1048 (unpublished data). These results suggest that cytotoxicity induced by S. aureus MVs is dependent on S. aureus strains, but not a common phenomenon in all S. aureus MVs. Bacterial effector molecules that induce cytotoxicity of host cells have not been determined yet.
S. aureus MVs are considered to be a potent initiator of immune responses. Although the composition of S. aureus MVs has not been fully characterized, a variety of PAMPs, including membrane proteins, peptidoglycans, lipoteichoic acid, and other bacteria-specific molecules, can be packaged in the S. aureus MVs, by which PAMPs packaged in the MVs interact with pattern-recognition receptors (PRRs) located on either the cytoplasmic membrane or the cytoplasm. Recognition of PAMPs by PRRs activates signal transduction cascades to initiate a defense response to fight invading bacteria. The MVs purified from S. aureus ATCC 14458 enhance the secretion of pro-inflammatory cytokines, including interleukin (IL)-6 and macrophage inflammatory protein (MIP)-1α, in mouse dermal fibroblasts (42). Moreover, MVs purified from a clinical MRSA isolate, S. aureus 06ST1048, also induce IL-1α, IL-6, IL-8, and MIP-1α in a transformed human keratinocytes, HaCaT cells (unpublished data). The silencing of Toll-like receptor (TLR) 2 and nucleotide-binding oligomerization domain-containing protein 2 (NOD2) genes by small interfering RNA results in the downregulation of proinflammatory cytokine genes in HaCaT cells treated with S. aureus MVs (unpublished data). This result indicates that diacylated lipoprotein and peptidoglycan packaged in the S. aureus MVs are responsible for the expression of proinflammatory cytokine genes, because TLR2 and NOD2 are the receptors for diacylated lipoprotein and peptidoglycan, respectively.
Atopic dermatitis (AD) is a chronic relapsing inflammatory skin disease with immunopathologic features that vary depending on the duration of the lesion (43~45). The AD lesions are highly susceptible to colonization or infection of S. aureus (46~48). S. aureus is isolated from ≥80% of skin lesions of AD patients. Although S. aureus infection is known to trigger skin inflammation and modulation of immune responses to exacerbate clinical AD, the underlying mechanisms by which S. aureus infection can worsen the AD lesions are not fully understood. Several S. aureus products, protein A, lipoteichoic acid, and peptidoglycan, are able to induce cytokine production and inflammatory responses in vitro assay (49), but the cutaneous application of these bacterial products does not induce major skin alterations that are observed in the AD lesions. Additional specific virulence factors or a complex of bacterial molecules are required for the development or worsening of AD.
Hong et al. (42) for the first time determined that S. aureus MVs are associated with the development of AD. They have demonstrated that S. aureus MVs produce proinflammatory mediators, including IL-6, MIP-1α, thymic stromal lymphopoietin, and eotaxin, in the cultured dermal fibroblasts. The application of S. aureus MVs in the tape-stripped mouse skin causes the thickening of epidermis and infiltration of mast cells and eosinophils in the dermis. After observing immunopathologies and skin lesions induced by S. aureus MVs, they concluded that S. aureus MVs could induce AD-like inflammation in the skin. Our team tried to determine whether S. aureus MVs were responsible for the worsening of AD. Mice were pretreated with the extract of house dust mite (Dermatophagoides farinae extract) for the development of AD and then applied S. aureus MVs in the skin lesions. S. aureus MVs exaggerate eczematous skin lesions, which are associated with a mixed Th1/Th2 immune response (unpublished data). Moreover, mice group treated with house dust mite and then S. aureus MVs shows a significant thickening of epidermis as compared to mice group treated with house dust mite. This result indicates that S. aureus MVs are directly responsible for the worsening of AD. Based on both experimental results, it is clear that S. aureus MVs are associated with the development and progression of AD.
The extracellular vesicle production is an evolutionally conserved process in both Gram-positive and Gram-negative bacteria. MVs derived from Gram-positive bacteria share common features with Gram-negative bacterial OMVs in terms of morphology, delivery mechanism to host cells, cytotoxicity, and immune modulation. Although the MV production and their roles in bacterial pathogenesis have not been studied in a variety of Gram-positive pathogens, MVs play a role in the delivery of bacterial components, including virulence determinants, to host cells, which are likely to be a good candidate to prevent or reduce disease development or progression. Future work in bacterial extracellular vesicles is likely to focus on the development of a novel therapeutics that blocks vesicle production in bacteria and vesicle delivery to host cells. Moreover, it is likely to develop MV vaccines against Gram-positive pathogens, since MVs are a complex of bacterial antigens.
Figures and Tables
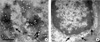 | Figure 1Membrane vesicle production in S. aureus. (A). Transmission electron micrograph (TEM) of MVs prepared from S. aureus ATCC 25923 cultured in Luria-Bertani broth. (B) Production and secretion of MVs from S. aureus 06ST1048 during in vivo infection. Mice were infected with S. aureus 06ST1048 intratracheally for 18 h. The infected lung tissues were prepared for TEM analysis. Arrows indicate MVs produced by S. aureus. |
References
1. Kuehn MJ, Kesty NC. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 2005. 19:2645–2655.

2. Ellis TN, Kuehn MJ. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol Mol Biol Rev. 2010. 74:81–94.

3. Beveridge TJ, Kadurugamuwa JL. Periplasm, periplasmic spaces, and their relation to bacterial wall structure: novel secretion of selected periplasmic proteins from Pseudomonas aeruginosa. Microb Drug Resist. 1996. 2:1–8.

4. Mashburn-Warren LM, Whiteley M. Special delivery: vesicle trafficking in prokaryotes. Mol Microbiol. 2006. 61:839–846.

5. Lee EY, Choi DS, Kim KP, Gho YS. Proteomics in gram-negative bacterial outer membrane vesicles. Mass Spectrom Rev. 2008. 27:535–555.

6. Wai SN, Lindmark B, Söderblom T, Takade A, Westermark M, Oscarsson J, et al. Vesicle-mediated export and assembly of pore-forming oligomers of the enterobacterial ClyA cytotoxin. Cell. 2003. 115:25–35.

7. Kesty NC, Kuehn MJ. Incorporation of heterologous outer membrane and periplasmic proteins into Escherichia coli outer membrane vesicles. J Biol Chem. 2004. 279:2069–2076.

8. Bomberger JM, Maceachran DP, Coutermarsh BA, Ye S, O'Toole GA, Stanton BA. Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog. 2009. 5:e1000382.
9. Kato S, Kowashi Y, Demuth DR. Outer membrane-like vesicles secreted by Actinobacillus actinomycetemcomitans are enriched in leukotoxin. Microb Pathog. 2002. 32:1–13.

10. Beveridge TJ. Structures of gram-negative cell walls and their derived membrane vesicles. J Bacteriol. 1999. 181:4725–4733.

11. Mayrand D, Grenier D. Biological activities of outer membrane vesicles. Can J Microbiol. 1989. 35:607–613.

12. Mashburn LM, Whiteley M. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature. 2005. 437:422–425.

13. Galka F, Wai SN, Kusch H, Engelmann S, Hecker M, Schmeck B, et al. Proteomic characterization of the whole secretome of Legionella pneumophila and functional analysis of outer membrane vesicles. Infect Immun. 2008. 76:1825–1836.

14. Kwon SO, Gho YS, Lee JC, Kim SI. Proteome analysis of outer membrane vesicles from a clinical Acinetobacter baumannii isolate. FEMS Microbiol Lett. 2009. 297:150–156.

15. Horstman AL, Kuehn MJ. Enterotoxigenic Escherichia coli secretes active heat-labile enterotoxin via outer membrane vesicles. J Biol Chem. 2000. 275:12489–12496.

16. Kesty NC, Mason KM, Reedy M, Miller SE, Kuehn MJ. Enterotoxigenic Escherichia coli vesicles target toxin delivery into mammalian cells. EMBO J. 2004. 23:4538–4549.

17. Kolling GL, Matthews KR. Export of virulence genes and Shiga toxin by membrane vesicles of Escherichia coli O157:H7. Appl Environ Microbiol. 1999. 65:1843–1848.

18. Lindmark B, Rompikuntal PK, Vaitkevicius K, Song T, Mizunoe Y, Uhlin BE, et al. Outer membrane vesicle-mediated release of cytolethal distending toxin (CDT) from Campylobacter jejuni. BMC Microbiol. 2009. 9:220.
19. Brandtzaeg P, Bryn K, Kierulf P, Ovstebø R, Namork E, Aase B, et al. Meningococcal endotoxin in lethal septic shock plasma studied by gas chromatography, mass-spectrometry, ultracentrifugation, and electron microscopy. J Clin Invest. 1992. 89:816–823.

20. Halhoul N, Colvin JR. The ultrastructure of bacterial plaque attached to the gingiva of man. Arch Oral Biol. 1975. 20:115–118.

21. Heczko U, Smith VC, Mark Meloche R, Buchan AM, Finlay BB. Characteristics of Helicobacter pylori attachment to human primary antral epithelial cells. Microbes Infect. 2000. 2:1669–1676.

22. Namork E, Brandtzaeg P. Fatal meningococcal septicaemia with "blebbing" meningococcus. Lancet. 2002. 360:1741.

23. Filipazzi P, Bürdek M, Villa A, Rivoltini L, Huber V. Recent advances on the role of tumor exosomes in immunosuppression and disease progression. Semin Cancer Biol. 2012. 22:342–349.

24. Raimondo F, Morosi L, Chinello C, Magni F, Pitto M. Advances in membranous vesicle and exosome proteomics improving biological understanding and biomarker discovery. Proteomics. 2011. 11:709–720.

25. Choi DS, Lee JM, Park GW, Lim HW, Bang JY, Kim YK, et al. Proteomic analysis of microvesicles derived from human colorectal cancer cells. J Proteome Res. 2007. 6:4646–4655.

26. Rodrigues ML, Nimrichter L, Oliveira DL, Frases S, Miranda K, Zaragoza O, et al. Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport. Eukaryot Cell. 2007. 6:48–59.

27. Rodrigues ML, Nakayasu ES, Oliveira DL, Nimrichter L, Nosanchuk JD, Almeida IC, et al. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot Cell. 2008. 7:58–67.

28. Dorward DW, Garon CF. DNA is packaged within membrane-derived vesicles of Gram-negative but not Gram-positive bacteria. Appl Environ Microbiol. 1990. 56:1960–1962.

29. Rivera J, Cordero RJ, Nakouzi AS, Frases S, Nicola A, Casadevall A. Bacillus anthracis produces membrane-derived vesicles containing biologically active toxins. Proc Natl Acad Sci U S A. 2010. 107:19002–19007.

30. Lee EY, Choi DY, Kim DK, Kim JW, Park JO, Kim S, et al. Gram-positive bacteria produce membrane vesicles: proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics. 2009. 9:5425–5436.

31. Crossley KB, Archer GL. The staphylococci in human disease. 1997. New York: Churchill Livingstone;682.
32. Groom AV, Wolsey DH, Naimi TS, Smith K, Johnson S, Boxrud D, et al. Community-acquired methicillin-resistant Staphylococcus aureus in a rural American Indian community. JAMA. 2001. 286:1201–1205.

33. Hiramatsu K, Cui L, Kuroda M, Ito T. The emergence and evolution of methicillin-resistant Staphylococcus aureus. Trends Microbiol. 2001. 9:486–493.

34. Tiwari HK, Sen MR. Emergence of vancomycin resistant Staphylococcus aureus (VRSA) from a tertiary care hospital from northern part of India. BMC Infect Dis. 2006. 6:156.
35. Gordon RJ, Lowy FD. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2008. 46:S350–S359.
36. Foster TJ, Höök M. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 1998. 6:484–488.
37. Tjalsma H, Antelmann H, Jongbloed JD, Braun PG, Darmon E, Dorenbos R, et al. Proteomics of protein secretion by Bacillus subtilis: separating the "secrets" of the secretome. Microbiol Mol Biol Rev. 2004. 68:207–233.

38. Gurung M, Moon DC, Choi CW, Lee JH, Bae YC, Kim J, et al. Staphylococcus aureus produces membrane-derived vesicles that induce host cell death. PLoS One. 2011. 6:e27958.
39. Kulp A, Kuehn MJ. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol. 2010. 64:163–184.

40. Ellis TN, Leiman SA, Kuehn MJ. Naturally produced outer membrane vesicles from Pseudomonas aeruginosa elicit a potent innate immune response via combined sensing of both lipopolysaccharide and protein components. Infect Immun. 2010. 78:3822–3831.

41. Jin JS, Kwon SO, Moon DC, Gurung M, Lee JH, Kim SI, et al. Acinetobacter baumannii secretes cytotoxic outer membrane protein A via outer membrane vesicles. PLoS One. 2011. 6:e17027.
42. Hong SW, Kim MR, Lee EY, Kim JH, Kim YS, Jeon SG, et al. Extracellular vesicles derived from Staphylococcus aureus induce atopic dermatitis-like skin inflammation. Allergy. 2011. 66:351–359.

43. Winkelmann RK, Rajka G. Atopic dermatitis and Hodgkin's disease. Acta Derm Venereol. 1983. 63:176–177.
45. Rajka G. Natural history and clinical manifestations of atopic dermatitis. Clin Rev Allergy. 1986. 4:3–26.

46. Hauser C, Wuethrich B, Matter L, Wilhelm JA, Sonnabend W, Schopfer K. Staphylococcus aureus skin colonization in atopic dermatitis patients. Dermatologica. 1985. 170:35–39.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download