Abstract
Mycobacterium abscessus (Mabc) is an emerging human pathogen. Less is known about the host immune response to Mabc than to M. tuberculosis. Here, we examined the intracellular signaling pathways that govern the expression of chemokines including (C-C motif) ligand 2 (CCL2) and (C-X-C motif) ligand 2 (CXCL2) in macrophages after infection with Mabc. Specifically, Mabc triggered the generation of reactive oxygen species (ROS) and the production of CCL2 and CXCL2 in murine bone marrow-derived macrophages (BMDMs). Mabc-induced CCL2, but not CXCL2, was dependent on the generation of ROS. Toll-like receptor (TLR) 2, MyD88, but not TRIF, was required for Mabc-induced CCL2 and CXCL2 expression. Additionally, Mabc infection significantly induced nuclear factor (NF)-κB nuclear translocation and luciferase activity. The activation of NF-κB was required for Mabc-induced CCL2, but not CXCL2 expression. Moreover, Mabc-induced ROS generation was required for NF-κB activation. Treatment of BMDMs with Mabc rapidly induced the activation of mitogen-activated protein kinase (MAPKs) pathways. Interestingly, CCL2 expression was dependent on the activation of JNK and ERK1/2 pathways, whereas it was negatively regulated by the p38 MAPK pathway. In contrast, Mabc-dependent CXCL2 expression was not regulated by MAPK pathways. These data suggest that intracellular ROS generation is required for innate and inflammatory responses during Mabc infection of macrophages.
Mycobacterium abscessus (Mabc), a rapidly growing mycobacterial species, is an emerging human pathogen that causes a variety of clinical syndromes. In immuno-compromised hosts, Mabc can result in localized cutaneous lesions to disseminated infection (1). Treatment of Mabc infection is problematic due to chemotherapy resistance and the requirement for long-term treatment (2). Control of mycobacterial infection required an appropriate immune response that limits bacterial spread and achieves homeostatic balance (3). The initial innate immune response plays an essential role in containment of the pathogen (4). Chemokines are involved in the host immune response through dendritic cell trafficking and maturation (5). Chemokines are secreted by activated macrophages and T cells. They attract various immune cells to the granuloma lesion, contributing to disease outcome (4, 6). In order to understand the host protective immune responses to Mabc, it is important to analyze how Mabc interacts with host innate receptors, and how it regulates the protective or inflammatory immune responses, including chemokine responses. The molecular mechanisms regarding Mabc-induction of host chemokine responses and modulation of intracellular regulatory mechanisms are unknown.
Innate immune cells respond to external pathogenic stimuli through multiple innate receptors. Through these interactions, innate immune responses are initiated and function as a primary defense against invading pathogens (7, 8). Toll-like receptors (TLRs) are critical innate immune receptors that play key roles in innate immunity through recognition of a variety of microbial pathogens (7~9). Two crucial TIR domain-containing adaptors, myeloid differentiation factor 88 (MyD88) and TIR domain-containing adaptor inducing interferon-β (TRIF), become activated upon ligation of TLRs and are responsible for MyD88-dependent and -independent pathways, respectively. During tuberculosis, TLRs 2, 4, and 9, and adaptor molecule MyD88 are known to be involved in protective roles against mycobacterial infection (4, 10). In addition, generation of intracellular reactive oxygen species (ROS) are critical for host defense mechanisms for bacterial killing and are involved in the activation of innate signaling pathways (11~13). Previous studies have shown that NADPH oxidase 2 (gp91phox/NOX2)-dependent signaling is involved in TLR-induced innate and inflammatory immune responses (14, 15). Although our previous studies have shown that Mabc can robustly induce proinflammatory cytokine responses in murine macrophages (16), the roles of TLR2, adaptor molecules, and intracellular ROS in the production of chemokines are unclear.
In TLR-dependent innate immune responses, two major intracellular signaling pathways, nuclear factor (NF)-κB and mitogen-activated protein kinases (MAPKs), play important roles in the activation of proinflammatory cytokine/chemokine production and antimicrobial protein generation, respectively. Recognition of microbial or danger patterns by TLRs trigger an intracellular signaling cascade that results in the activation of NF-κB, which leads to the expression of inflammatory cytokines and mediators (7, 9). MAPKs, a family of serine/threonine protein kinases, can be activated in response to external stress including mycobacteria (8). Bacterial pathogens target and manipulate kinase pathways for their benefit (18, 19). The activation of MAPK signaling pathways can lead to the synthesis of inflammatory cytokine production, making them therapeutic targets for a variety of inflammatory and infectious diseases (8, 17).
In this study, we examined the roles of intracellular signaling pathways including ROS, TLR2, MyD88, TRIF, NF-κB, and MAPKs in the activation of chemokine generation by bone marrow-derived macrophages (BMDMs) after Mabc infection. We found that the expression of TLR2 and MyD88 are required for Mabc-induced expression of chemokines including chemokine (C-C motif) ligand 2 (CCL2) and (C-X-C motif) ligand 2 (CXCL2) by BMDMs. Additionally, we found that Mabc-induced ROS and NF-κB activation contributes to the induction of CCL2, but not CXCL2, expression in BMDMs. Further, Mabc-induced CCL2 expression was regulated by the extracellular signal-regulated protein kinases (ERK) 1/2 and c-Jun N-terminal kinase (JNK) pathways, whereas it was negatively regulated by the p38 MAPK pathway. However, Mabc-dependent CXCL2 expression was not regulated by either of the three MAPK subfamilies.
Mabc (ATCC 19977) were grown as previously described (16). Briefly, Mabc was grown in Middlebrook 7H10 agar medium (Difco Laboratories, Detroit, MI, USA) supplemented with 10% OADC (BD Pharmingen; San Diego, CA, USA) and 0.05% Tween 80 (Sigma-Aldrich, St. Louis, MO, USA) at 37℃. Collected Mabc were resuspended in Middlebrook 7H9 medium (Difco Laboratories) supplemented with 10% OADC, 0.2% glycerol, and 0.05% Tween 80 for 2 weeks, and then aliquots were stored at -70℃ until use. Mabc were thawed, colony forming units (cfu) were determined, and the known concentrations of Mabc were grown for 7 days (37℃, 5% CO2). Single-cell suspensions of Mabc were prepared as described previously (16).
C57BL/6 mice were purchased from DBL (Chungbuk, Korea). TLR2, Myd88, and Trif knockout (KO) mice were kindly provided by Dr. S. Akira (Osaka University, Osaka, Japan). BMDMs were isolated from 6-week-old mice and differentiated for 5 days in medium containing 20 µg/ml macrophage colony-stimulating factor (M-CSF; R&D Systems, Minneapolis, MN, USA), as described previously (15). Culture medium consisted of DMEM (Lonza, Walkersville, MD, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Lonza), 50 U/ml penicillin, and 50 µg/ml streptomycin. This study was approved by the Animal Care and Use Committee of Chungnam National University.
Lipopolysaccharides (LPS; Escherichia coli 0111:B4) and synthetic bacterial lipopeptide (Pam3Cys-Ser-Lys4-OH) were from Invivogen (San Diego, CA, USA). N-acetylcysteine (NAC), diphenylene iodonium (DPI), BAY 11-7082, caffeic acid phenethyl ester (CAPE), U0126, SB203580, and SP600125 were from Calbiochem (San Diego, CA, USA). 4,5-dihydroxy-1,3-benzene disulfonic acid-disodium salt (Tiron) and dimethyl sulfoxide (DMSO; added to the cultures at 0.05% (v/v) as a solvent control) were from Sigma-Aldrich. Phospho-SAPK/JNK (Thr183/Tyr185), phospho-ERK1/2 (Thr202/Tyr204), phospho-p38 (Thr180/Tyr182), and phospho-IκB kinase (IKK)-α/β (Ser176/180) were from Cell signaling Technology (Cell signaling, Beverly, MA, USA). IκBα (C-21) and β-actin (I-19) were from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
For Western blot analysis, cell lysate was boiled in sample buffer and separated by 12% SDS-PAGE gels. Proteins were transferred to polyvinyldifluoride membranes (PVDF; Millipore, Boston, MA, USA). Abs against p-SAPK/JNK, p-ERK1/2, p-p38, p-IKKα/β, and IκBα were diluted at a ratio of 1:1,000. Membranes were developed by ECL solution (Millipore; Danvers, MA, USA) and exposed to chemiluminescence film (Fujifilm, Japan). ELISA analysis was performed as described previously (16). The cytokine levels measured were mouse CCL2 (BD PharMingen) and mouse CXCL2 (R&D Systems).
For semi-quantitative RT-PCR analysis, total RNA was extracted from cells using TRIzol (Invitrogen; Carlsbad, CA, USA), as described previously (16). Primer sequences were as follows: mCcl2 (forward: 5'-ACTCAAGCCAGCTCTCTCTT-3', reverse: 5'-TTCCTTCTTGGGGTCAGCAC-3'), mCxcl2 (forward: 5'-GAACAAAGGCAAGGCTAACT-3', reverse: 5'-AACATAACAACATCTGGGCAAT-3'), β-actin (forward: 5'-TCATGAAGTGTGACGTTGACATCCGT-3', reverse: 5'-CCTAGAAGCATTTGCGGTGCACGATG-3'). Annealing was performed at 56℃ for 45 s. RT-PCR products were visualized on 1.5% agarose gels and stained with ethidium bromide. Densitometry values were normalized to β-actin mRNA levels.
Intracellular superoxide levels were measured as described previously (16). Briefly, BMDMs were infected with Mabc (moi = 3, 30 min) in the presence or absence of NADPH oxidase inhibitor (DPI; 45 min). Cells were washed three times in PBS and incubated with either 10 µm dihydroethidium (DHE; Calbiochem) for 30 min, followed by analysis with a FACSCanto flow cytometer (Becton Dickinson, San Jose, CA, USA). Analysis of 50,000 to 100,000 events per sample was quantified by FlowJo software (Tree Star; Ashland, OR, USA).
To assay for nuclear translocation of NF-κB p65, BMDMs were infected with Mabc (moi = 3) for 30 min and fixed with 4% paraformaldehyde in PBS for 10 min at room temperature (RT). Cells were permeabilized with 0.25% Triton X-100 in PBS for 15 min at RT. Cells were immunostained with primary Ab (rabbit anti-mouse NF-κB p65, 1:400 dilution, for 2 h; Santa Cruz) and secondary Ab (anti-rabbit Alexa Fluro 488, 1:400 dilution, for 1 h; Invitrogen) at RT. Nuclei were visualized by incubation with 1 µg/ml DAPI (Sigma). Unbound Ab was removed with PBS, and cells were imaged using a Zeiss LSM510 META confocal microscope (Germany).
NF-κB luciferase reporter assays were performed as described previously (20). BMDMs were transduced with a NF-κB-Luc reporter plasmid (Genetransfer Vector Core; Iowa City, IA, USA) for 36 h, and infected with Mabc for 6 h. Infected cells were harvested and washed three times in PBS, and then lysed in luciferase lysis buffer (Promega; Madison, WI, USA). A luciferase assay system (Promega) was used according to the manufacturer's instructions. Results were normalized to protein content of three independent extracts.
Previous studies reported that Mabc activate the production of proinflammatory cytokines, including tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), in BMDMs (16). We first examined whether Mabc infection of BMDMs induced the generation of ROS and production of CCL2 and CXCL2. Treatment of BMDMs with Mabc (moi = 3) significantly induced generation of ROS, which peaked at 30 min after infection (Fig. 1A). Mabc-induced superoxide generation was rapidly and completely blocked by pre-treatment with the NADPH oxidase inhibitor DPI (Fig. 1A), suggesting that Mabc-induced superoxide production is mediated through NADPH oxidase-dependent ROS.
We further examined the time-dependent mRNA and protein expression of CCL2 and CXCL2 in BMDMs after infection with Mabc. As shown in Fig. 1B, Mabc stimulation with BMDMs activated CCL2 and CXCL2 mRNA expression, which peaked at 6 h after infection. Additionally, peak protein levels of Ccl2 and Cxcl2 were observed in BMDMs 18 and 48 h after Mabc infection, respectively (Fig. 1C). These data suggest that Mabc treatment of BMDMs actively induced ROS generation and the expression of CCL2 and CXCL2 mRNA and protein.
We next investigated the roles of intracellular ROS in the expression of CCL2 and CXCL2 in BMDMs. To examine this, we pre-treated BMDMs with antioxidants NAC, DPI, and Tiron before Mabc infection. mRNA and proteins were collected from cell lysates and supernatants after 6 and 18 h of infection, respectively. As shown in Fig. 2A and B, pre-treatment of ROS scavengers significantly abolished Mabc-induced CCL2 mRNA and protein production in a dose-dependent manner. However, Mabc-induced CXCL2 mRNA and protein expression was not inhibited by pre-treatment of cells with ROS blockers (Fig. 2A and B). Together, these data suggest that Mabc-induced CCL2, but not CXCL2, production is dependent on intracellular ROS generation.
We previously reported that TLR2 is required for Mabc-induced TNF-α and IL-6 expression in BMDMs (16). However, whether Mabc-induced CCL2 and CXCL2 production is mediated through TLR2 has not been investigated. Moreover, the roles of MyD88 and TRIF in chemokine production during Mabc infection are unknown. Thus, we performed ELISA analysis of supernatants collected from Mabc-infected BMDMs from wild-type, TLR2 KO, MyD88 KO, and TRIF KO mice.
Consistent with our previous study (16), Mabc-induced CCL2 and CXCL2 protein synthesis was largely suppressed in TLR2-deficient BMDMs (Fig. 3A). However, after Mabc treatment, basal levels of CCL2 and CXCL2 remained in TLR2-deficient BMDMs for 18 h (Fig. 3A). We then examined the levels of Mabc-induced chemokine production in MyD88- or TRIF-deficient BMDMs. Notably, Mabc-induced CCL2 and CXCL2 production was nearly completely abrogated in MyD88-, but not TRIF-deficient, BMDMs (Fig. 3B). We found that LPS-induced TNF-α and chemokine production was not decreased in TLR2-deficient BMDMs, whereas it was partially reduced in MyD88- and TRIF-deficient BMDMs (Fig. 3B and data not shown). Additionally, Pam3CSK4-dependent TNF-α and chemokine production was completely attenuated in TLR2- and MyD88-deficient BMDMs (Fig. 3B and data not shown). Together, Mabc-induced CXCL2 and CCL2 production was dependent on the expression of TLR2 and MyD88, but not TRIF, in BMDMs.
Because NF-κB activation is involved in TLR downstream signaling pathways (9), we hypothesized that Mabc infection drives the activation of NF-κB promoter activities. To examine a possible role for Mabc in NF-κB activation, we performed a NF-κB luciferase assay in BMDMs transduced with adenovirus encoding a luciferase reporter plasmid containing response elements for NF-κB (Ad-NF-κB-Luc). As shown in Fig. 4A, Mabc stimulation significantly increased NF-κB reporter gene activities in BMDMs transduced with Ad-NF-κB-Luc, in a moi-dependent manner. There was no significant increase in NF-κB promoter activities in cells transduced with control adenovirus (data not shown).
We also determined that the NF-κB p65 subunit translocated to the nucleus after Mabc infection. In BMDMs infected with Mabc, the nuclear translocation of NF-κB p65 is rapid, reaching maximal nuclear localization by 30 min (Fig. 4B, bottom). The time-dependent appearance of NF-κB p65 in the nucleus was visualized by immunofluorescence microscopy and quantified by image analysis. Fig. 4B shows that treatment of BMDMs with Mabc rapidly activated nuclear translocation of NF-κB p65, which peaked 30 min after infection.
Following TLR ligand engagement, the activation of the IKK-α/β complex, consisting of NEMO and IKKα/β, is important for TLR signaling activation through catalyzing IκB proteins for phosphorylation and degradation (21). To examine whether Mabc infection led to the activation of IKK-α/β and IκBα, Western blot analysis was performed to detect Ser 176/180 phosphorylation of IKKα/β and degradation of IκBα in BMDMs after Mabc stimulation. Fig. 4C shows that Mabc infection of BMDMs rapidly induced Ser 176/180 phosphorylation of IKKα/β and degradation of IκBα starting at 5 min after stimulation. Peak levels of Ser 176/180 phosphorylation of IKKα/β and degradation of IκBα were detected in BMDMs around 15 to 30 min after Mabc infection. These data suggest that Mabc infection rapidly induces NF-κB activation, translocation, phosphorylation of IKKα/β, and degradation of IκBα in BMDMs.
Next we examined involvement of the NF-κB pathway in the production of CCL2 and CXCL2 in BMDMs treated with Mabc. Fig. 5A showed that the induction of mRNA expression of Ccl2, but not Cxcl2, in response to Mabc was abrogated in a dose-dependent fashion when BMDMs were pretreated with Bay11-7082 or CAPE, specific inhibitors of the NF-κB signalling pathway (22). Accordingly, Mabc-induced CCL2, but not CXCL2, was impaired by a NF-κB inhibitor (Fig. 5B).
We also investigated an interaction between NF-κB and ROS signaling pathways. When BMDMs were transduced with Ad-NF-κB-Luc, followed by pre-treatment with an antioxidant and Mabc stimulation, Mabc-induced NF-κB luciferase activities were significantly decreased in a dose-dependent fashion by pre-treatment with ROS blockers (Fig. 5C). These results indicate that ROS-dependent NF-κB signaling is essential for Mabc-induced CCL2, but not CXCL2, in BMDMs.
Our previous studies showed that Mabc activates MAPKs, p38 MAPK, and ERK1/2 pathways in BMDMs (16). We determined whether Mabc-induced MAPK pathways were required for the regulation of CCL2 and CXCL2 production in BMDMs. We sought to determine the effect of Mabc on the responsiveness of BMDMs in terms of MAPK activation. BMDMs were infected with Mabc and activation of MAPKs was analyzed. Mabc exerted strong activation of JNK, p38 MAPK, and ERK1/2 phosphorylation at 15 to 30 min (Fig. 6A).
We next determined the role of MAPKs in the induction of CCL2 and CXCL2. Pre-treatment of BMDMs with an inhibitor of p38 MAPK (SB203580) showed increased CCL2 production upon stimulation with Mabc (Fig. 6B). However, Mabc-induced CCL2 production was inhibited by a selective JNK inhibitor (SP600125) and an ERK1/2 inhibitor (U0126) (Fig. 6B). In contrast, inhibitors of all 3 MAPK subfamilies had no effect on Mabc-induced CXCL2 secretion in BMDMs. Together, these data suggest that Mabc-induced CCL2, but not CXCL2, production is mediated through a JNK- and ERK1/2-dependent pathway. Additionally, Mabc-induced CCL2 was negatively regulated by p38 MAPK in BMDMs.
Chemokines, a family of low molecular weight proteins classified by the presence of a cysteine motif in the N-terminal region of the protein, play critical roles in regulation of immune responses and inflammation through direction of leukocyte trafficking and positioning to disease sites (5). During tuberculosis, multiple chemokines and chemokine receptors are involved in protective immune responses and pathogenesis (6, 8, 23~25). A recent study also reported that, in the early phase of murine tuberculosis, CXC chemokines CXCL1, CXCL5, and CXCL9, are induced to promote the recruitment of neutrophils to the lungs infected with M. tuberculosis (26). Additionally, levels of C-C chemokines, including CCL-2 (MCP-1), CCL8 (MCP-2), CCL12 (MCP-5), and CCL22 (MDC), are increased between D15 and D21 in mice after M. tuberculosis infection (26, 27). Although chemokine production is involved in the recruitment of monocytes/macrophages during M. tuberculosis infection (26, 27), the induction and regulatory mechanisms of chemokine expression in macrophages after atypical mycobacterial infection are largely unknown. In this study, we showed that Mabc infection of macrophages rapidly and strongly activated CCL2 and CXCL2, and that multiple signaling pathways, including ROS, TLR2, MyD88, TRIF, NF-κB, and MAPKs, play a role in the differential regulation of CCL2 and CXCL2 expression.
Mabc can activate macrophage inflammatory responses through TLR2 and the c-type lectin Dectin-1 (16). We found that Mabc induced the production of CCL2 and CXCL2 through TLR2-MyD88 pathways. However, the TRIF pathway did not play a role in Mabc-induced chemokine expression in macrophages. In M. tuberculosis infection, MyD88 and TRIF play an important role as crucial messengers in activation of downstream kinases, including the IKK complex and MAPKs, to induce the production of cytokines, chemokines, and innate immune effecters (9, 21, 28). Earlier studies showed that TLR2 and MyD88 play a critical role in inflammatory responses and protective immunity against M. tuberculosis infection (29, 30). Together with our current data, the TLR2-MyD88 pathway is involved in protective immune responses in macrophages in response to M. tuberculosis and atypical mycobacteria. The role of Dectin-1 and chemokine expression during Mabc infection will be examined in future studies.
Intracellular ROS signaling through the mitochondrial respiratory chain and NADPH oxidase activity is crucial for maintenance of redox homeostasis and activation of membrane receptor signaling, such as growth factor-mediated signaling pathways (14, 31, 32). During M. tuberculosis infection, crosstalk between NOX2 and TLR2 may play an essential role in the generation of NF-κB-dependent proinflammatory cytokine responses in macrophages (15). Our findings show that Mabc-induced CCL2, but not CXCL2, production is significantly attenuated in macrophages by inhibitors of ROS-NF-κB signaling. Distinct roles for ROS-NF-κB signaling in CCL2 and CXCL2 synthesis suggest that other signaling pathways may also participate in Mabc-induced CXCL2. Future investigation is required to assess other signaling mediators/pathways in Mabc-mediated CXCL2 production.
Additionally, our data suggest that an ROS signaling pathway is an upstream regulator in NF-κB signaling. Previous studies have shown that TLR4 signaling activation by hyaluronic acid fragments induces NADPH oxidase-dependent ROS generation, which promotes NF-κB-dependent inflammatory molecules during skin inflammation (33). Highly sulfated polysaccharide carrageenan-dependent inflammatory cascades and ROS generation are associated with phosphorylation of IKK. These signaling pathways are integrated at the level of the IKK signalosome (34). Our previous studies also indicated that intracellular ROS signaling pathways are involved in NF-κB signaling pathways and antimicrobial protein cathelicidin expression in TLR-induced inflammation and mycobacterial infection (18, 35). Thus, we hypothesize that NF-κB-mediated induction of proinflammatory and innate immune mediator expression results from a protective host immune response to inflammatory/oxidative stress by triggering redox signaling pathways.
MAPK pathways are involved in protective and regulatory functions of host defense against mycobacterial infections (8, 36). Previous studies showed that MAPK pathways are activated in murine macrophages by infection with M. tuberculosis and atypical mycobacteria, and that they are involved in the generation of proinflammatory cytokines (15, 16). It was also reported that Mabc and M. avium induce TNF-α production, which is dependent on the p38 MAPK pathway (37). Recent studies showed that M. tuberculosis inhibits interferon-γ-induced protein 10 (IP-10) and macrophage-induced gene (MIG), both of which are associated with host defense against mycobacteria and are regulated by the p38 MAPK-dependent pathway (38). However, current data show that CXCL2 and CCL2 are distinctly regulated by three families of MAPKs: Mabc-induced CCL2 secretion is dependent on JNK and ERK1/2, but not the p38 MAPK pathway; Mabc-induced CXCL2 is independent on three families of MAPKs. These data suggest that TLR2-dependent NF-κB and MAPK pathways are required for the expression of Mabc-induced CCL2, but not CXCL2, in macrophages.
Figures and Tables
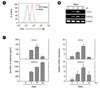 | Figure 1Mabc induce intracellular ROS generation and production of CCL2 and CXCL2 in BMDMs. (A) BMDMs were infected with Mabc (moi = 3) for 30 min in the absence or presence of DPI (10 µM, 45 min). Cells were incubated with DHE (10 µM) for 30 min and subjected to FACS analysis for superoxide production. (B and C) BMDMs were infected with Mabc (moi = 3) for the indicated times. (B) Cells were harvested (0~18 h) and subjected to semi-quantitative RT-PCR analysis of Ccl2 and Cxcl2 mRNA expression. Top, representative gel images. Bottom, densitometry values for Ccl2 and Cxcl2 were normalized to β-actin mRNA levels. Data are from a representative of three independent experiments. (C) Supernatants were harvested (0~48 h) and subjected to ELISA analysis for CCL2 and CXCL2 production. The results are the means ± SD of three independent experiments. U, uninfected; Mabc, M. abscessus. |
 | Figure 2Intracellular ROS formation is involved in Mabc-induced chemokine expression in BMDMs. BMDMs were infected with Mabc (moi = 3) in the presence or absence of antioxidant (NAC; 10, 20 or 30 mM), NADPH oxidase inhibitor (DPI; 1, 5 or 10 µM), or Tiron (10, 20 or 30 mM) for 45 min. (A) After 6 h, the cells were harvested and subjected to semi-quantitative RT-PCR analysis of Ccl2 and Cxcl2 mRNA expression. Top, representative gel images. Bottom, densitometry values for Ccl2 and Cxcl2 were normalized to β-actin mRNA levels. Data are from a representative of three independent experiments. (B) After 18 h, the supernatants were harvested and subjected to ELISA analysis of CCL2 and CXCL2 production. The results are the means ± SD of three independent experiments. *p < 0.05, **p < 0.01, **p < 0.001 compared with control cultures. U, uninfected; Mabc, M. abscessus; D, solvent control (0.05% DMSO). |
 | Figure 3TLR2 and MyD88, but not TRIF, are essential for Mabc-induced chemokine production in BMDMs. BMDMs from (A) TLR2 WT and TLR2 KO or (B) WT, MyD88 KO, and TRIF KO mice were infected with Mabc (moi = 3) for 18 h. The supernatants were harvested and subjected to ELISA analysis of CCL2 and CXCL2 production. The results are the means ± SD of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001 compared with each stimulator (Mabc, LPS, Pam3CSK4). U, uninfected; L, LPS; P, Pam3CSK4. |
 | Figure 4Mabc rapidly induces NF-κB activation in BMDMs. (A) BMDMs were transduced with a NF-κB-Luc reporter plasmid for 36 h, and infected with Mabc (moi = 1, 3 or 9) for 6 h. The cells were harvested and luciferase reporter activity was determined. Luciferase activity results are representative of three independent experiments. (B) BMDMs were infected with Mabc (moi = 3) for 30 min and immunostained with anti-NF-κB p65 and anti-rabbit-Alexa Fluor 488. Top, representative immunofluorescence images. Scale bars = 20 µm. Bottom, relative fluorescence intensities are presented as the means ± SD of three independent samples, with each experiment including at least 200 cells scored in six random fields. (C) BMDMs were infected with Mabc (moi = 3) for the indicated times (0~120 min). The cells were harvested and subjected to Western blot analysis for phosphorylated IKK-α/β and IκBα. β-actin was probed as a loading control. The results are the means ± SD of three independent experiments. U, uninfected; Mabc, M. abscessus
|
 | Figure 5NF-κB and Mabc-induced chemokine expression. (A and B) BMDMs were infected with Mabc (moi = 3) in presence or absence of BAY 11-7082 (BAY; 0.3, 1 or 3 µM, 45 min) or CAPE (1, 5 or 10 µM, 2 h). (A) After 6 h, cells were harvested and subjected to semi-quantitative RT-PCR analysis for detection of Ccl2 and Cxcl2 mRNA expression. (B) After 18 h, the supernatants were harvested and subjected to ELISA analysis of CCL2 and CXCL2 production. (C) BMDMs were transduced with NF-κB-Luc reporter plasmid for 36 h. Cells were infected with Mabc (moi = 3) for 6 h in presence or absence of NAC (10, 20 or 30 mM), DPI (1, 5 or 10 µM), or Tiron (10, 20 or 30 mM) for 45 min. Cells were harvested and luciferase reporter activity was determined. Luciferase activity results are representative of three independent experiments. The results are the means ± SD of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001 compared with control cultures. U, uninfected; D, solvent control (0.05% DMSO). |
 | Figure 6MAPK phosphorylation and Mabc-induced chemokine expression. (A) BMDMs were infected with Mabc (moi = 3) for the indicated times (0~480 min). Cells were harvested and subjected to Western blot analysis of phosphorylated MAPK (JNK, ERK1/2, and p38). β-actin was probed as a loading control. (B) BMDMs were infected with Mabc (moi = 3) for 18 h in presence or absence of JNK inhibitor (SP600125; 5, 20 or 30 µM), MEK-1 inhibitor (U0126; 5, 10 or 20 µM), or p38 inhibitor (SB203580; 1, 5 or 10 µM) for 45 min. The supernatants were harvested and subjected to ELISA analysis of CCL2 and CXCL2 production. The results are the means ± SD of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001 compared with control cultures. U, uninfected; SP, SP600125; SB, SB203580; D, solvent control (0.05% DMSO). |
References
1. Medjahed H, Gaillard JL, Reyrat JM. Mycobacterium abscessus: a new player in the mycobacterial field. Trends Microbiol. 2010. 18:117–123.
2. Petrini B. Mycobacterium abscessus: an emerging rapid-growing potential pathogen. APMIS. 2006. 114:319–328.

3. Huynh KK, Joshi SA, Brown EJ. A delicate dance: host response to mycobacteria. Curr Opin Immunol. 2011. 23:464–472.

4. Jo EK. Mycobacterial interaction with innate receptors: TLRs, C-type lectins, and NLRs. Curr Opin Infect Dis. 2008. 21:279–286.

5. Bachmann MF, Kopf M, Marsland BJ. Chemokines: more than just road signs. Nat Rev Immunol. 2006. 6:159–164.

6. Natarajan K, Kundu M, Sharma P, Basu J. Innate immune responses to M. tuberculosis infection. Tuberculosis (Edinb). 2011. 91:427–431.
7. Doyle SL, O'Neill LA. Toll-like receptors: from the discovery of NFkappaB to new insights into transcriptional regulations in innate immunity. Biochem Pharmacol. 2006. 72:1102–1113.

8. Jo EK, Yang CS, Choi CH, Harding CV. Intracellular signalling cascades regulating innate immune responses to Mycobacteria: branching out from Toll-like receptors. Cell Microbiol. 2007. 9:1087–1098.

10. Kleinnijenhuis J, Oosting M, Joosten LA, Netea MG, Van Crevel R. Innate immune recognition of Mycobacterium tuberculosis. Clin Dev Immunol. 2011. 2011:405310.
11. Bogdan C, Röllinghoff M, Diefenbach A. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr Opin Immunol. 2000. 12:64–76.

12. Kohchi C, Inagawa H, Nishizawa T, Soma G. ROS and innate immunity. Anticancer Res. 2009. 29:817–821.
13. Steevels TA, Meyaard L. Immune inhibitory receptors: essential regulators of phagocyte function. Eur J Immunol. 2011. 41:575–587.

14. Bae YS, Oh H, Rhee SG, Yoo YD. Regulation of reactive oxygen species generation in cell signaling. Mol Cells. 2011. 32:491–509.

15. Yang CS, Shin DM, Kim KH, Lee ZW, Lee CH, Park SG, et al. NADPH oxidase 2 interaction with TLR2 is required for efficient innate immune responses to mycobacteria via cathelicidin expression. J Immunol. 2009. 182:3696–3705.

16. Shin DM, Yang CS, Yuk JM, Lee JY, Kim KH, Shin SJ, et al. Mycobacterium abscessus activates the macrophage innate immune response via a physical and functional interaction between TLR2 and dectin-1. Cell Microbiol. 2008. 10:1608–1621.

17. Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy--from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta. 2005. 1754:253–262.

18. Yang CS, Lee JS, Song CH, Hur GM, Lee SJ, Tanaka S, et al. Protein kinase C zeta plays an essential role for Mycobacterium tuberculosis-induced extracellular signal-regulated kinase 1/2 activation in monocytes/macrophages via Toll-like receptor 2. Cell Microbiol. 2007. 9:382–396.

19. Krachler AM, Woolery AR, Orth K. Manipulation of kinase signaling by bacterial pathogens. J Cell Biol. 2011. 195:1083–1092.

20. Lee HM, Yuk JM, Shin DM, Yang CS, Kim KK, Choi DK, et al. Apurinic/apyrimidinic endonuclease 1 is a key modulator of keratinocyte inflammatory responses. J Immunol. 2009. 183:6839–6848.

21. Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010. 11:373–384.

22. Kim E, Kim SH, Kim S, Kim TS. The novel cytokine p43 induces IL-12 production in macrophages via NF-kappaB activation, leading to enhanced IFN-gamma production in CD4+ T cells. J Immunol. 2006. 176:256–264.

23. Jo EK, Park JK, Dockrell HM. Dynamics of cytokine generation in patients with active pulmonary tuberculosis. Curr Opin Infect Dis. 2003. 16:205–210.

24. Lee JS, Lee JY, Son JW, Oh JH, Shin DM, Yuk JM, et al. Expression and regulation of the CC-chemokine ligand 20 during human tuberculosis. Scand J Immunol. 2008. 67:77–85.

25. Elkington PT, D'Armiento JM, Friedland JS. Tuberculosis immunopathology: the neglected role of extracellular matrix destruction. Sci Transl Med. 2011. 3:71ps6.

26. Kang DD, Lin Y, Moreno JR, Randall TD, Khader SA. Profiling early lung immune responses in the mouse model of tuberculosis. PLoS One. 2011. 6:e16161.

27. Kipnis A, Basaraba RJ, Orme IM, Cooper AM. Role of chemokine ligand 2 in the protective response to early murine pulmonary tuberculosis. Immunology. 2003. 109:547–551.

28. Jeong E, Lee JY. Intrinsic and extrinsic regulation of innate immune receptors. Yonsei Med J. 2011. 52:379–392.

29. Underhill DM, Ozinsky A, Smith KD, Aderem A. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc Natl Acad Sci U S A. 1999. 96:14459–14463.

30. Fremond CM, Togbe D, Doz E, Rose S, Vasseur V, Maillet I, et al. IL-1 receptor-mediated signal is an essential component of MyD88-dependent innate response to Mycobacterium tuberculosis infection. J Immunol. 2007. 179:1178–1189.

31. Rhee SG. Redox signaling: hydrogen peroxide as intracellular messenger. Exp Mol Med. 1999. 31:53–59.

32. Park HS, Lee SH, Park D, Lee JS, Ryu SH, Lee WJ, et al. Sequential activation of phosphatidylinositol 3-kinase, beta Pix, Rac1, and Nox1 in growth factor-induced production of H2O2. Mol Cell Biol. 2004. 24:4384–4394.

33. Kwon MJ, Han J, Kim BH, Lee YS, Kim TY. Superoxide dismutase 3 suppresses hyaluronic acid fragments mediated skin inflammation by inhibition of toll-like receptor 4 signaling pathway: superoxide dismutase 3 inhibits reactive oxygen species-induced trafficking of toll-like receptor 4 to lipid rafts. Antioxid Redox Signal. 2012. 16:297–313.

34. Bhattacharyya S, Dudeja PK, Tobacman JK. Carrageenan-induced NFkappaB activation depends on distinct pathways mediated by reactive oxygen species and Hsp27 or by Bcl10. Biochim Biophys Acta. 2008. 1780:973–982.

35. Lee HM, Shin DM, Choi DK, Lee ZW, Kim KH, Yuk JM, et al. Innate immune responses to Mycobacterium ulcerans via toll-like receptors and dectin-1 in human keratinocytes. Cell Microbiol. 2009. 11:678–692.

36. Schorey JS, Cooper AM. Macrophage signalling upon mycobacterial infection: the MAP kinases lead the way. Cell Microbiol. 2003. 5:133–142.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download