Abstract
Vibrio vulnificus produces Hemolysin/cytolysin (VvhA), which is one of the most potent exotoxins capable of killing mice at submicrogram levels. However, V. vulnificus growth and vvhA expression are severely repressed and extracellular VvhA produced at low levels is easily inactivated in human body fluids. This study was conducted to obtain additional unequivocal evidence of the enigmatic characteristic of VvhA. V. vulnificus growth was stimulated, vvhA expression was de-repressed, and extracellular VvhA production was increased in cirrhotic ascites, a human ex vivo experimental system, by a mutation of fur encoding ferric uptake regulator, which acts as a transcriptional repressor. However, regardless of the presence or absence of the fur mutation, extracellular VvhA activity was not detected in cirrhotic ascites. These results indicate that VvhA is easily inactivated even when vvhA expression and extracellular VvhA production are maintained at high levels in cirrhotic ascites.
Go to : 
REFERENCES
1). Jones MK., Oliver JD. Vibrio vulnificus: disease and pathogenesis. Infect Immun. 2009. 77:1723–33.
2). Gray LD., Kreger AS. Purification and characterization of an extracellular cytolysin produced by Vibrio vulnificus. Infect Immun. 1985. 48:62–72.
3). Wright AC., Morris JG Jr. The extracellular cytolysin of Vibrio vulnificus: inactivation and relationship to virulence in mice. Infect Immun. 1991. 59:192–7.
4). Kim YR., Lee SE., Kook H., Yeom JA., Na HS., Kim SY, et al. Vibrio vulnificus RTX toxin kills host cells only after contact of the bacteria with host cells. Cell Microbiol. 2008. 10:848–62.
5). Kook H., Lee SE., Baik YH., Chung SS., Rhee JH. Vibrio vulnificus hemolysin dilates rat thoracic aorta by activating guanylate cyclase. Life Sci. 1996. 59::p. L41–7.
6). Kwon KB., Yang JY., Ryu DG., Rho HW., Kim JS., Park JW, et al. Vibrio vulnificus cytolysin induces super-oxide anion-initiated apoptotic signaling pathway in human ECV304 cells. J Biol Chem. 2001. 276:47518–23.
7). Lee SE., Ryu PY., Kim SY., Kim YR., Koh JT., Kim OJ, et al. Production of Vibrio vulnificus hemolysin in vivo and its pathogenic significance. Biochem Biophys Res Commun. 2004. 324:86–91.
8). Choi MH., Park RY., Sun HY., Kim CM., Bai YH., Lee SE, et al. Suppression and inactivation of Vibrio vulnificus hemolysin in cirrhotic ascites, a human ex vivo experimental system. FEMS Immunol Med Microbiol. 2006. 47:226–32.
9). Kim CM., Chung YY., Shin SH. Iron differentially regulates gene expression and extracellular secretion of Vibrio vulnificus cytolysin-hemolysin. J Infect Dis. 2009. 200:582–9.
10). Reddy GP., Hayat U., Abeygunawardana C., Fox C., Wright AC., Maneval DR Jr, et al. Purification and determination of the structure of capsular polysaccharide of Vibrio vulnificus M06-24. J Bacteriol. 1992. 174:2620–30.
11). Kim SY., Lee SE., Kim YR., Kim CM., Ryu PY., Choy HE, et al. Regulation of Vibrio vulnificus virulence by the LuxS quorum-sensing system. Mol Microbiol. 2003. 48:1647–64.
12). Kim CM., Park RY., Park JH., Sun HY., Bai YH., Ryu PY, et al. Vibrio vulnificus vulnibactin, but not metallo-protease VvpE, is essentially required for iron-uptake from human holotransferrin. Biol Pharm Bull. 2006. 29:911–8.
13). Leong SA., Neilands JB. Siderophore production by phytopathogenic microbial species. Arch Biochem Biophys. 1982. 218:351–9.

15). Miller JH. A short course in bacterial genetics; A Laboratory Manual and Handbook for Escherichia coli and related bacteria. New York: Cold Spring Harbor Laboratory Press;1992.
16). Rioux C., Jordan DC., Rattray JBM. Colorimetric determination of catechol siderophores in microbial cultures. Anal Biochem. 1983. 133:163–9.

17). Fan JJ., Shao CP., Ho YC., Yu CK., Hor LI. Isolation and characterization of a Vibrio vulnificus mutant deficient in both extracellular metalloprotease and cytolysin. Infect Immun. 2001. 69:5943–8.
18). Brennt CE., Wright AC., Dutta SK., Morris JG Jr. Growth of Vibrio vulnificus in serum from alcoholics: association with high transferrin iron saturation. J Infect Dis. 1991. 164:1030–2.
19). Bullen JJ., Sparding PB., Ward CG., Gutteridge JM. Hemochromatosis, iron and septicemia caused by Vibrio vulnificus. Arch Intern Med. 1991. 151:1606–9.
20). Hor LI., Chang TT., Wang ST. Survival of Vibrio vulnificus in whole blood from patients with chronic liver diseases: association with phagocytosis by neutrophils and serum ferritin levels. J Infect Dis. 1999. 179:275–8.
21). Hor LI., Chang YK., Chang CC., Lei HY., Ou JT. Mechanisms of high susceptibility of iron-overloaded mouse to Vibrio vulnificus infection. Microbiol Immunol. 2000. 44:871–8.
22). Kim CM., Park RY., Choi MH., Sun HY., Shin SH. Ferrophilic characteristics of Vibrio vulnificus and potential usefulness of iron chelation therapy. J Infect Dis. 2007. 195:90–8.
23). Webster AC., Litwin CM. Cloning and characterization of vuuA, a gene encoding the Vibrio vulnificus ferric vulnibactin receptor. Infect Immun. 2000. 68:526–34.
24). Bang YB., Lee SE., Rhee JH., Choi SH. Evidence that expression of the Vibrio vulnificus hemolysin gene is dependent on cyclic AMP and cyclic AMP-receptor protein. J Bacteriol. 1999. 181:7639–42.
25). Choi HK., Park NY., Kim DI., Chung HJ., Ryu S., Choi SH. Promoter analysis and regulatory characteristics of vvhBA encoding cytolytic hemolysin of Vibrio vulnificus. J Biol Chem. 2002. 277:47292–9.
26). Park KH., Yang HB., Kim HG., Lee YR., Hur H., Kim JS, et al. Low density lipoprotein inactivates Vibrio vulnificus cytolysin through the oligomerization of toxin monomer. Med Microbiol Immunol. 2005. 194:137–41.
Go to : 
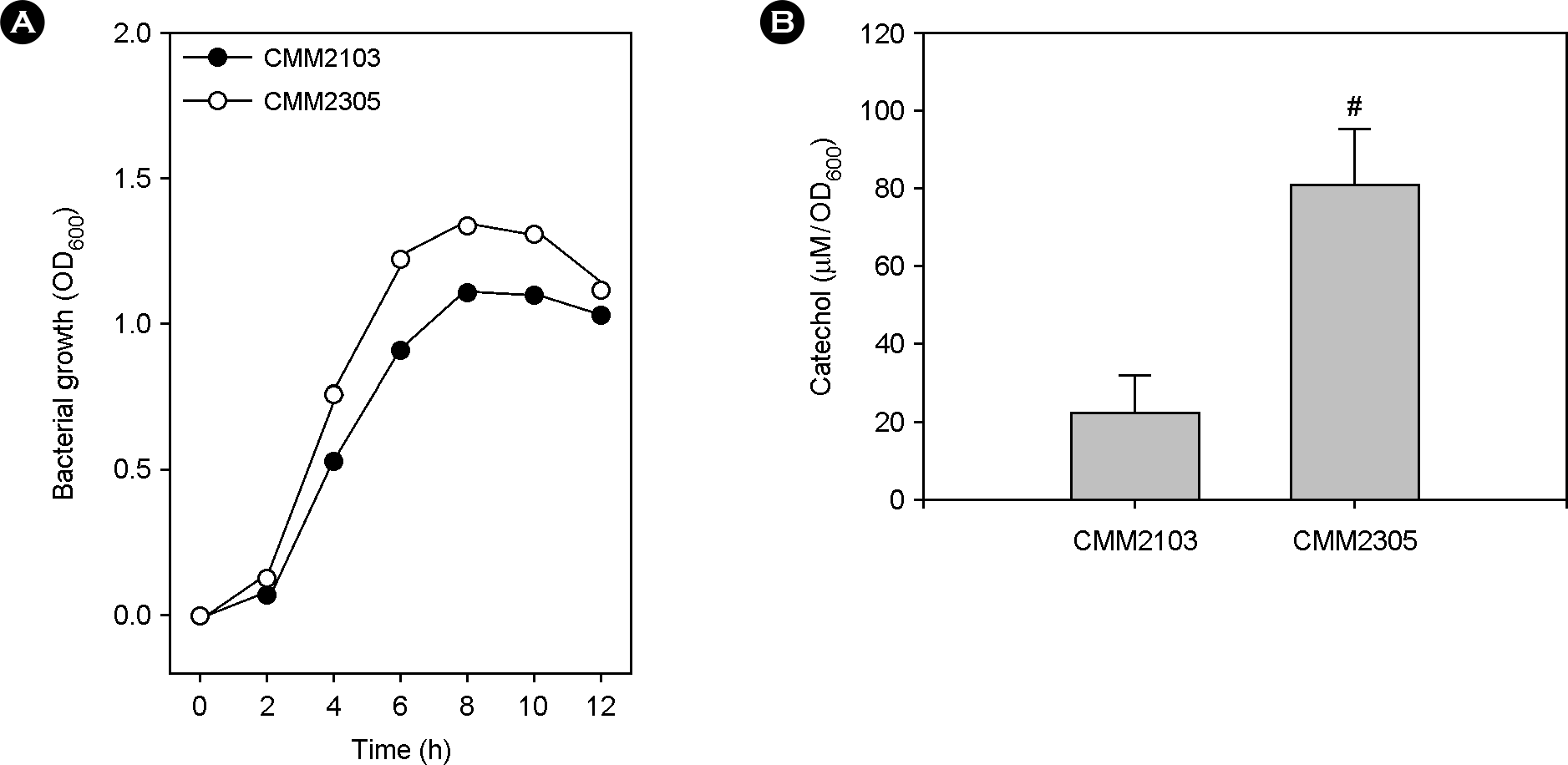 | Figure 1.Effect of a fur mutation on the production of catechol-siderophore vulnibactin. The two PvvhA::lacZ transcription reporter strains, CMM2103 with wild-type fur and CMM2305 with deleted fur, were cultured in iron-deficient Heart Infusion broths at 37°C for 12 h. (A) Bacterial growth was measured by the optical density of culture aliquots at 600 nm (OD600). (B) Culture supernatants were obtained by centrifugation and catechol concentration in the culture supernatants was determined by the Arnow test. Numeric values are expressed as the means ± standard deviation, from triplicate measurements. The # symbol indicates a statistically significant difference between the two means (p < 0.05 in Student's t-test). |
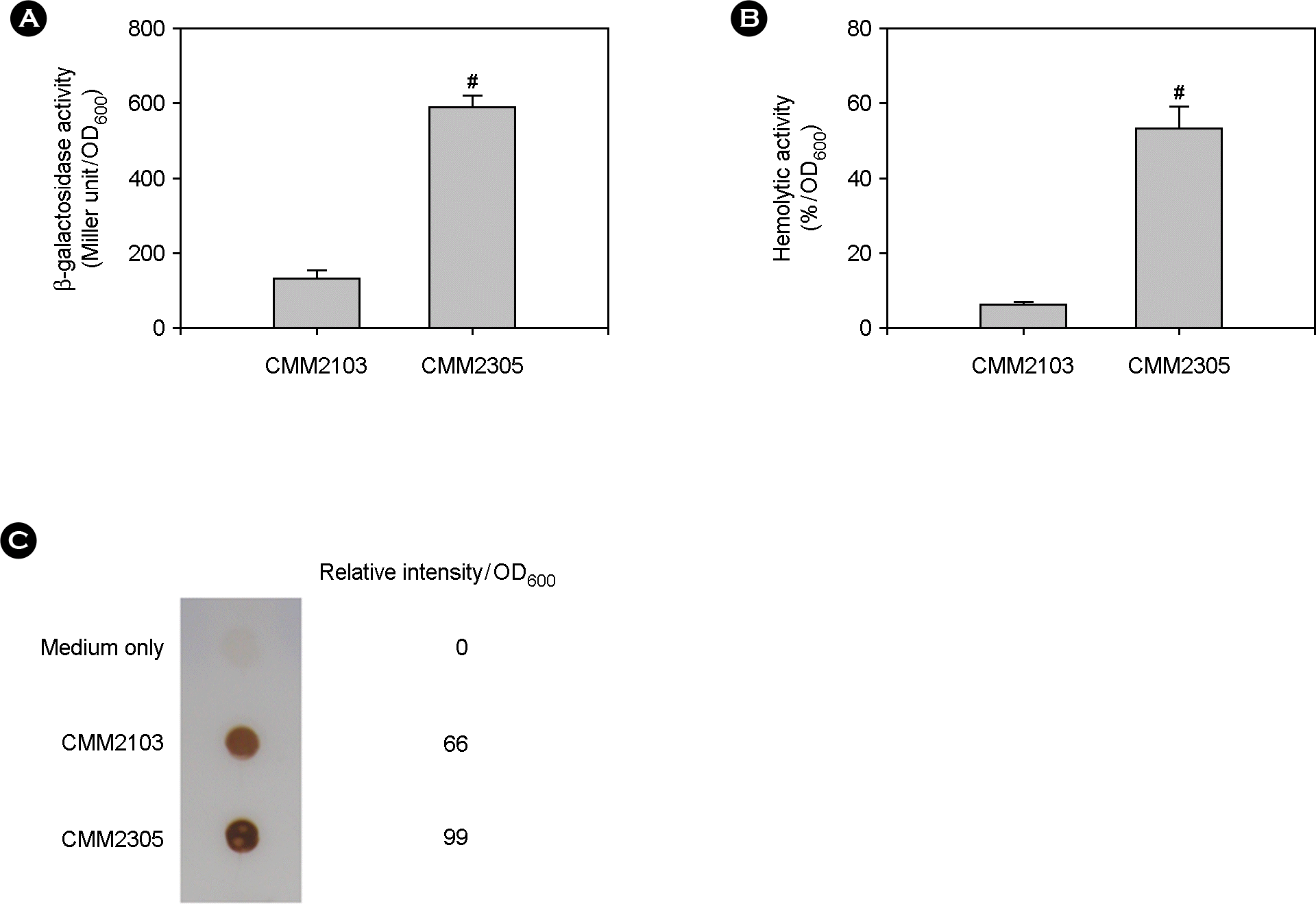 | Figure 2.Effect of a fur mutation on vvhA expression and extracellular VvhA production and activity. The two PvvhA::lacZ transcription reporter strains, CMM2103 with wild-type fur and CMM2305 with deleted fur, were cultured in iron-deficient Heart Infusion broths at 37°C for 12 h. Bacterial growth was measured by the optical density of culture aliquots at 600 nm (OD600), and culture supernatants were obtained by centrifugation. (A) The vvhA expression levels were measured by the Miller method and β-galactosidase activity was expressed as Miller units. (B) Extracellular VvhA activity was measured by the tube hemolytic assay using a 1% human red blood cell suspension. (C) Extracellular VvhA amount was determined by the dot blot method. For comparison on a per cell basis, signal intensities were quantified by densitometry. Numeric values are expressed as the means ± standard deviation, from triplicate measurements. The # symbol indicates a statistically significant difference between the two means (p < 0.05 in Student's t-test). |
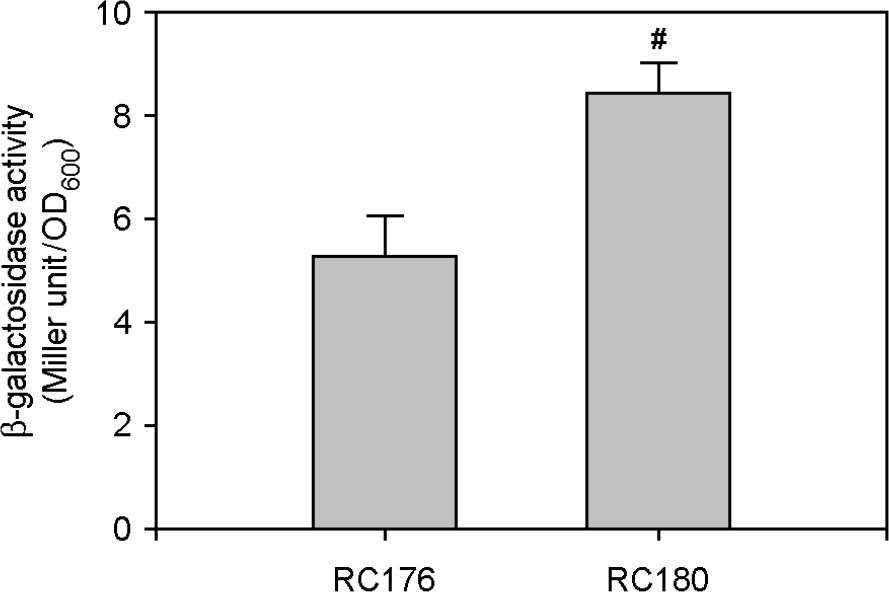 | Figure 3.Effect of a fur mutation on pilD expression. The two PpilD::lacZ transcription reporter strains, RC176 with wild-type fur and RC180 with deleted fur, were cultured in iron-deficient Heart Infusion broths at 37°C for 12 h. Bacterial growth was measured by the optical density of culture aliquots at 600 nm (OD600), and culture supernatants were obtained by centrifugation. (A) The pilD expression levels were measured by the Miller method and β-galactosidase activity was expressed as Miller units. Numeric values are expressed as the means ± standard deviation, from triplicate measurements. The # symbol indicates a statistically significant difference between the two means (p < 0.05 in Student's t-test). |
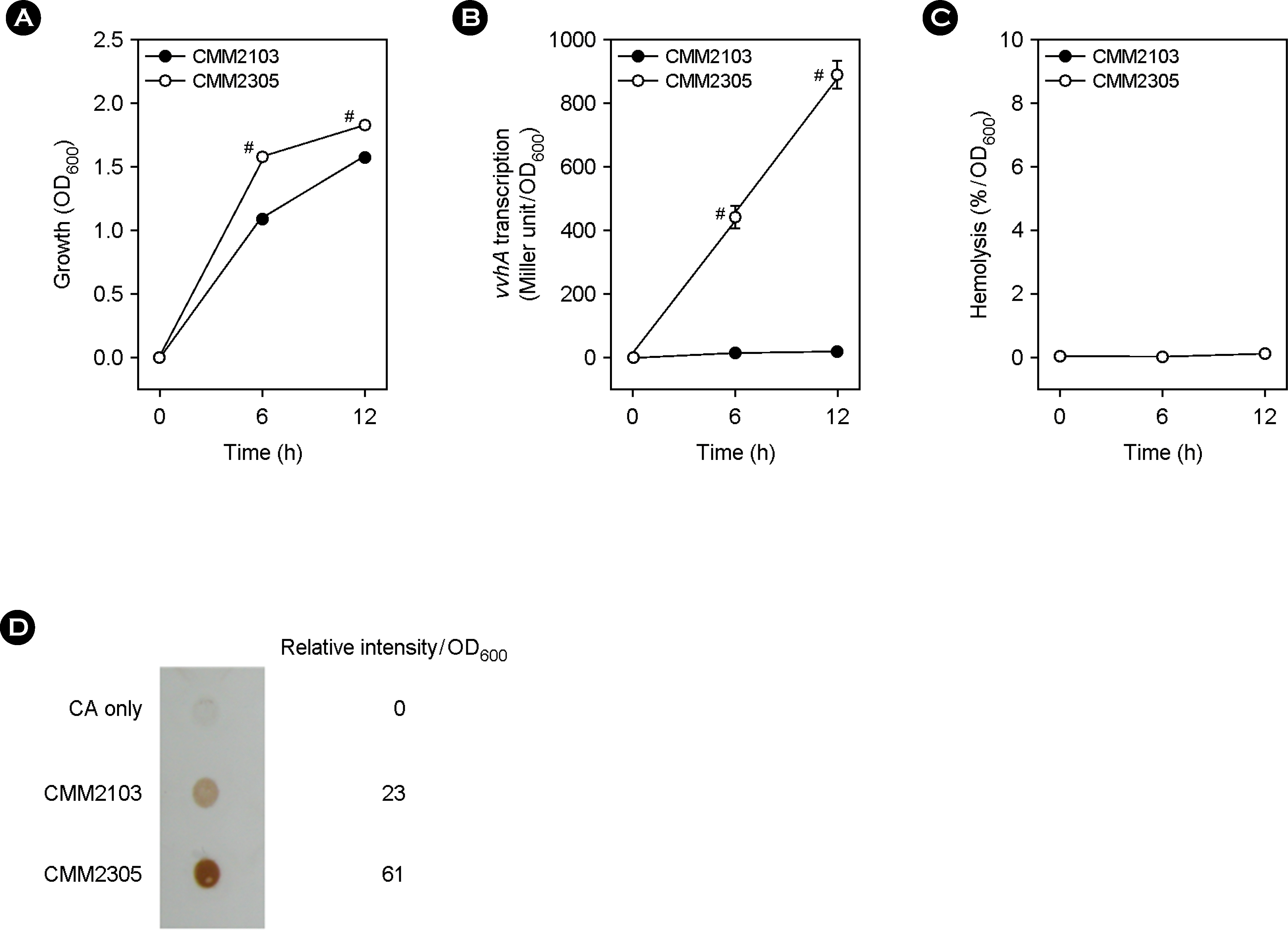 | Figure 4.Effect of a fur mutation on vvhA expression and extracellular VvhA production and activity in cirrhotic ascites. The two PvvhA::lacZ transcription reporter strains, CMM2103 with wild-type fur and CMM-2305 with deleted fur, were cultured in cirrhotic ascites at 37°C for 12 h. (A) Bacterial growth was measured by the optical density of culture aliquots at 600 nm (OD600). (B) The vvhA expression levels in culture aliquots were measured by the Miller method and β-galactosidase activity was expressed as Miller units. (C) Extracellular VvhA activity in culture supernatants was measured by the tube hemolytic assay using a 1% human red blood cell suspension. (D) Extracellular VvhA amount in culture supernatants was determined by the dot blot method. For comparison on a per cell basis, signal intensities were digitalized by densitometry. Numeric values are expressed as the means ± standard deviation, from triplicate measurements. The # symbol indicates a statistically significant difference between the two means (p < 0.05 in Student's t-test). |
Table 1.
Bacterial strains used in this study




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download