Abstract
Oral microorganisms, including pathogens together with commensals, interact with oral epithelial cells, which can lead to the activation and expression of a variety of inflammatory mediators in epithelial cells. Fusobacterium nucleatum is a filamentous human pathogen that is strongly associated with periodontal diseases. Our previous data suggest that Weissella cibaria, an oral commensal, inhibits the proliferation of periodontopathic bacteria including F. nucleatum. The aim of this study was to examine the effects of W. cibaria on the inflammatory mediators, interleukin (IL)-6 and IL-8, in KB cells stimulated by F. nucleatum. In a reverse transcription-polymerase chain reaction and an enzyme-linked immunosorbent assay, live F. nucleatum alone induced high levels of gene expression and protein release of IL-6 and IL-8, whereas W. cibaria alone did not induce IL-6 and IL-8 responses in KB cells. W. cibaria dose-dependently inhibited the increases of the IL-6 and IL-8 gene expression as well as IL-6 protein level in KB cells which was induced by F. nucleatum. Bacterial viability and its coaggregation with F. nucleatum are not essential in the inhibitory effect of W. cibaria. Visible effects of W. cibaria on the attachment and invasion of KB cells by F. nucleatum were observed. In conclusion, W. cibaria may exert immunomodulatory effects on the IL-6 and IL-8 responses to F. nucleatum-activated KB cells.
Go to : 
REFERENCES
1). Haffajee AD., Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1994. 5:78–111.

2). Slots J., Ting M. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in human periodontal disease: occurrence and treatment. Periodontol 2000. 1999. 20:82–121.
3). Haffajee AD., Teles RP., Socransky SS. Association of Eubacterium nodatum and Treponema denticola with human periodontitis lesions. Oral Microbiol Immunol. 2006. 21:269–82.
4). Bolstad AI., Jensen HB., Bakken V. Taxonomy, biology, and periodontal aspects of Fusobacterium nucleatum. Clin Microbiol Rev. 1996. 9:55–71.
5). Han YW., Shi W., Huang GT., Kinder Haake S., Park NH., Kuramitsu H, et al. Interactions between periodontal bacteria and human oral epithelial cells: Fusobacterium nucleatum adheres to and invades epithelial cells. Infect Immun. 2000. 68:3140–6.
6). Kagnoff MF., Eckmann L. Epithelial cells as sensors for microbial infection. J Clin Invest. 1997. 100:6–10.

7). Okada H., Murakami S. Cytokine expression in periodontal health and disease. Crit Rev Oral Biol Med. 1998. 9:248–66.

8). Birkedal-Hansen H. Role of cytokines and inflammatory mediators in tissue destruction. J Periodontal Res. 1993. 28:500–10.

10). Irwin CR., Myrillas TT. The role of IL-6 in the pathogenesis of periodontal disease. Oral Dis. 1998. 4:43–7.

11). Yumoto H., Nakae H., Fujinaka K., Ebisu S., Matsuo T. Interleukin-6 (IL-6) and IL-8 are induced in human oral epithelial cells in response to exposure to periodontopathic Eikenella corrodens. Infect Immun. 1999. 67:384–94.
12). Sfakianakis A., Barr CE., Kreutzer D. Mechanisms of Actinobacillus actinomycetemcomitans-induced expression of interleukin-8 in gingival epithelial cells. J Periodontol. 2001. 72:1413–9.
13). Takashiba S., Naruishi K., Murayama Y. Perspective of cytokine regulation for periodontal treatment: fibroblast biology. J Periodontol. 2003. 74:103–10.

14). Frieling J., Mulder JA., Hendriks T., Curfs JH., van der Linden CJ., Sauerwein RW. Differential induction of pro- and anti-inflammatory cytokines in whole blood by bacteria: effects of antibiotic treatment. Antimicrob Agents Chemother. 1997. 41:1439–43.

15). Neish AS., Gewirtz AT., Zeng H., Young AN., Hobert ME., Karmali V, et al. Prokaryotic regulation of epithelial responses by inhibition of IkappaB-alpha ubiquitination. Science. 2000. 289:1560–3.
16). Kelly D., Campbell JI., King TP., Grant G., Jansson EA., Coutts AG, et al. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-gamma and RelA. Nat Immunol. 2004. 5:104–12.
17). Bjorkroth KJ., Schillinger U., Geisen R., Weiss N., Hoste B., Holzapfel WH, et al. Taxonomic study of Weissella confusa and description of Weissella cibaria sp. nov., detected in food and clinical samples. Int J Syst Evol Microbiol. 2002. 52:141–8.
18). Kang MS., Chung J., Kim SM., Yang KH., Oh JS. Effect of Weissella cibaria isolates on the formation of Streptococcus mutans biofilm. Caries Res. 2006. 40:418–25.
19). Kang MS., Kim BG., Chung J., Lee HC., Oh JS. Inhibitory effect of Weissella cibaria isolates on the production of volatile sulphur compounds. J Clin Periodontol. 2006. 33:226–32.
20). Kang MS., Lim HS., Kim SM., Lim YJ., Lee HC., Oh JS. Quantitative analysis of Weissella cibaria against periodontopathic bacteria by real-time PCR. J Bacteriol Virol. 2009. 39:295–305.
21). Kang MS., Na HS., Oh JS. Coaggregation ability of Weissella cibaria isolates with Fusobacterium nucleatum and their adhesiveness to epithelial cells. FEMS Microbiol Lett. 2005. 253:323–9.
22). Edwards AM., Grossman TJ., Rudney JD. Fusobacterium nucleatum transports noninvasive Streptococcus cristatus into human epithelial cells. Infect Immun. 2006. 74:654–62.
23). Kang IC., Kuramitsu HK. Induction of monocyte chemoattractant protein-1 by Porphyromonas gingivalis in human endothelial cells. FEMS Immunol Med Microbiol. 2002. 34:311–7.
24). Na YJ., Jeon YJ., Suh JH., Kang JS., Yang KH., Kim HM. Suppression of IL-8 gene expression by radicicol is mediated through the inhibition of ERK1//2 and p38 signaling and negative regulation of NF-kappaB and AP-1. Int Immunopharmacol. 2001. 1:1877–87.
25). Deshpande RG., Khan MB., Genco CA. Invasion of aortic and heart endothelial cells by Porphyromonas gingivalis. Infect Immun. 1998. 66:5337–43.
26). Walker C., Ratliff D., Muller D., Mandell R., Socransky SS. Medium for selective isolation of Fusobacterium nucleatum from human periodontal pockets. J Clin Microbiol. 1979. 10:844–9.
27). Kang MS., Oh JS., Kang IC., Hong SJ., Choi CH. Inhibitory effect of methyl gallate and gallic acid on oral bacteria. J Microbiol. 2008. 46:744–50.

28). Kornman KS., Page RC., Tonetti MS. The host response to the microbial challenge in periodontitis: assembling the players. Periodontol 2000. 1997. 14:33–53.

29). Kjeldsen M., Holmstrup P., Bendtzen K. Marginal periodontitis and cytokines: a review of the literature. J Periodontol. 1993. 64:1013–22.

30). Wilson M., Reddi K., Henderson B. Cytokine-inducing components of periodontopathic bacteria. J Periodontal Res. 1996. 31:393–407.
31). Tonetti MS., Imboden MA., Gerber L., Lang MP., Laissue J., Mueller C. Localized expression of mRNA for phagocyte-specific chemotactic cytokines in human periodontal infections. Infect Immun. 1994. 62:4005–14.

32). Gamonal J., Acevedo A., Bascones A., Jorge O., Silva A. Characterization of cellular infiltrate, detection of chemokine receptor CCR5 and interleukin-8 and RANTES chemokines in adult periodontitis. J Periodontal Res. 2001. 36:194–203.

34). Zhang L., Li N., Caicedo R., Neu J. Alive and dead Lactobacillus rhamnosus GG decrease tumor necrosis factor-alpha induced interleukin-8 production in Caco-2 cells. J Nutr. 2005. 135:1752–6.
35). Huang GT., Kim D., Lee JK., Kuramitsu HK., Haake SK. Interleukin-8 and intercellular adhesion molecule 1 regulation in oral epithelial cells by selected periodontal bacteria: multiple effects of Porphyromonas gingivalis via antagonistic mechanisms. Infect Immun. 2001. 69:1364–72.
Go to : 
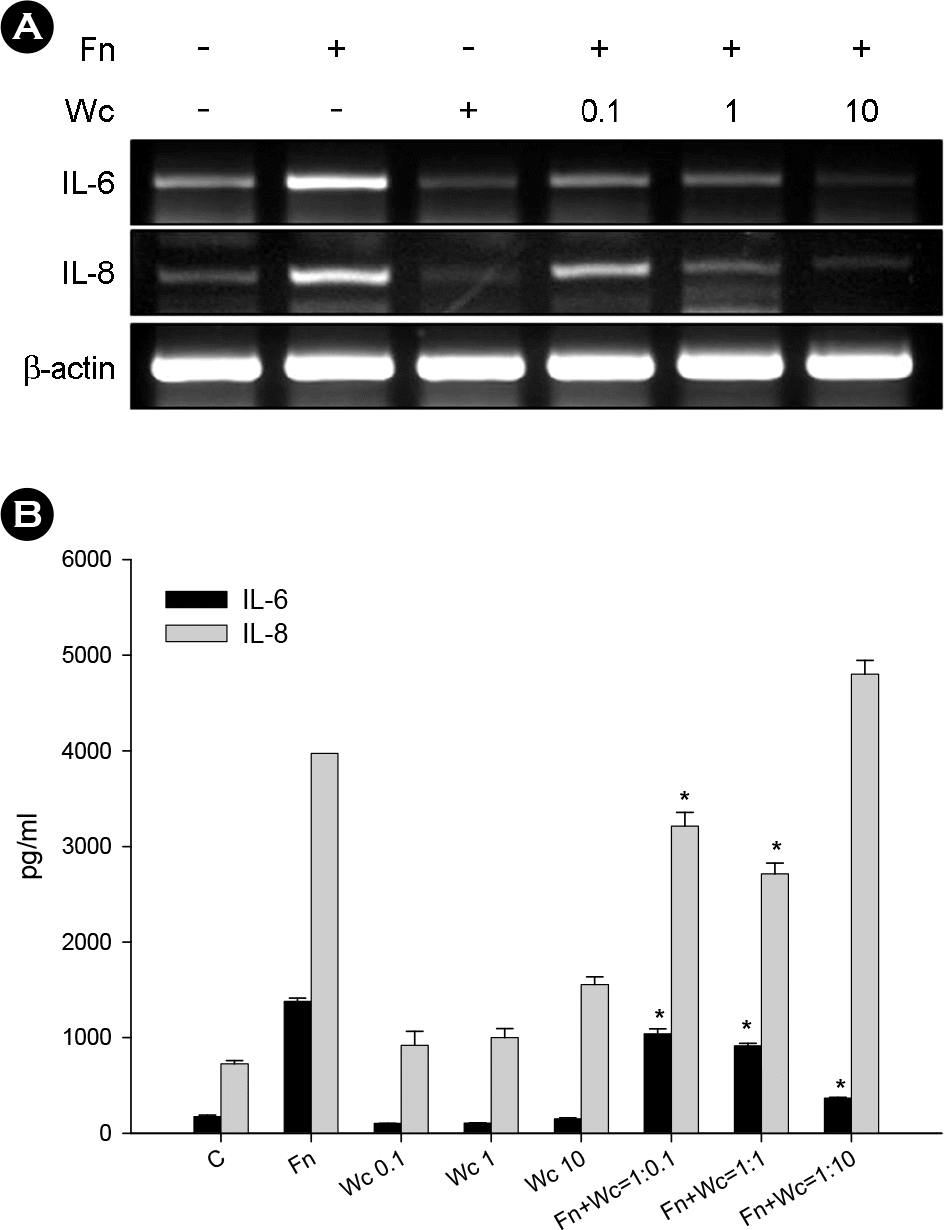 | Figure 1.Dose responses of W. cibaria on F. nucleatum-induced IL-6 and IL-8 production in KB cells. IL-6 and IL-8 mRNA (A) and protein (B) were assayed by RT-PCR and ELISA, respectively. F. nucleatum was used at a MOI of 100. W. cibaria was used at different MOI, from 10 to 1,000 (which are relatively expressed as 0.1, 1, or 10 W. cibaria per F. nucleatum). The data is expressed with the mean ± SD of a representative experiment performed in triplicate. C, control cells without stimulation; Fn, cells stimulated with F. nucleatum; Wc, cells stimulated with W. cibaria; Fn+Wc, F. nucleatum with W. cibaria. ∗p < 0.05, compared with F. nucleatum alone. |
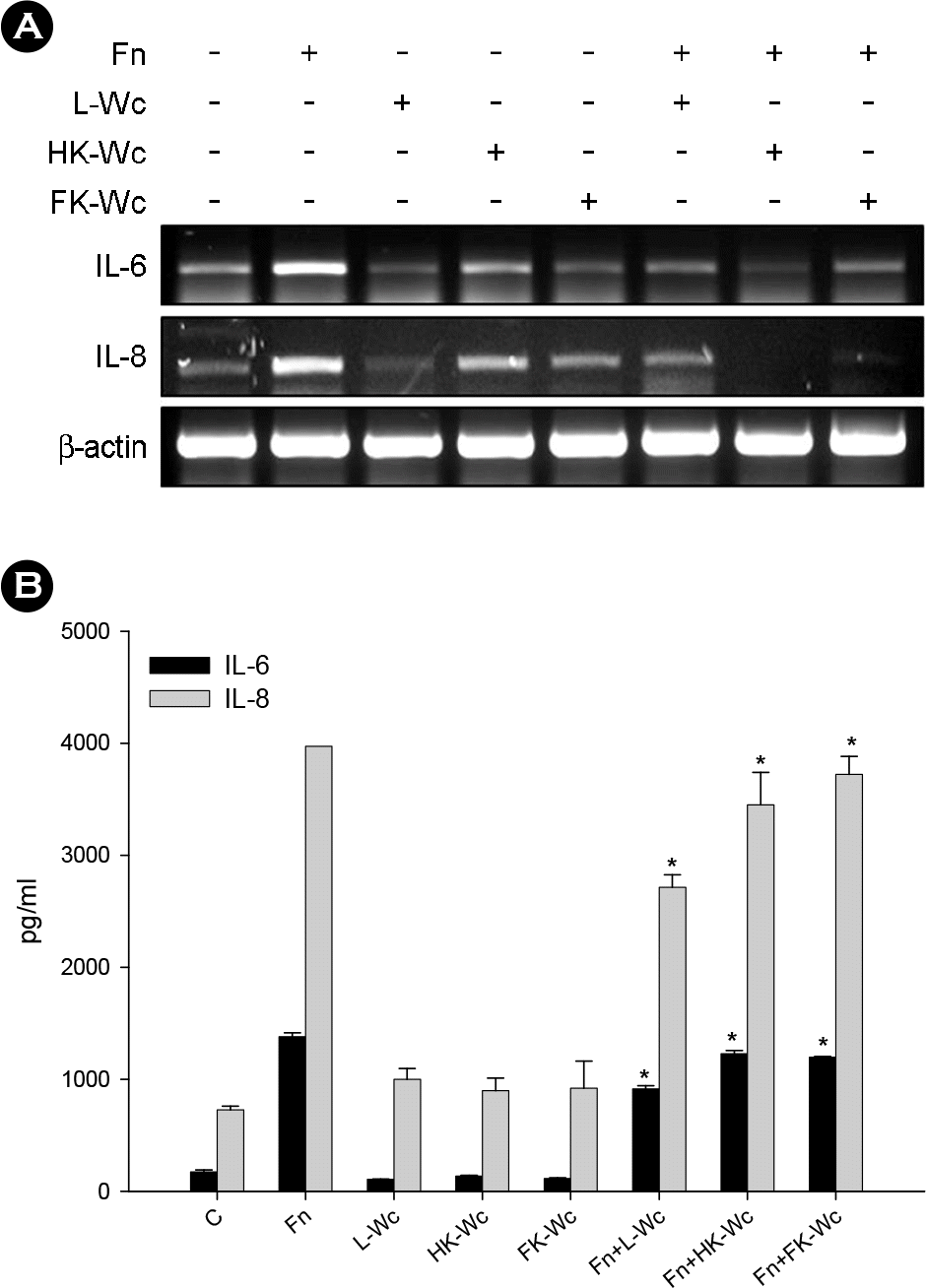 | Figure 2.Effects of live and killed W. cibaria on F. nucleatum-induced IL-6 and IL-8 production in KB cells. IL-6 and IL-8 mRNA (A) and protein (B) were assayed by RT-PCR and ELISA, respectively. KB cells were stimulated at an F. nucleatum to live, heat-killed, or formalin-killed W. cibaria ratio of 1:1, as described in Materials and Methods. The data is expressed with the mean ± SD of a representative experiment performed in triplicate. C, control cells without stimulation; Fn, F. nucleatum; L-Wc, live W. cibaria; HK-Wc, heat-killed W. cibaria; FK, formalin-killed W. cibaria; Fn+L-Wc, F. nucleatum with live W. cibaria; Fn+HK-Wc, F. nucleatum with heat-killed W. cibaria; Fn+FK-Wc, F. nucleatum with formalin-killed W. cibaria. ∗p < 0.05, compared with F. nucleatum alone. |
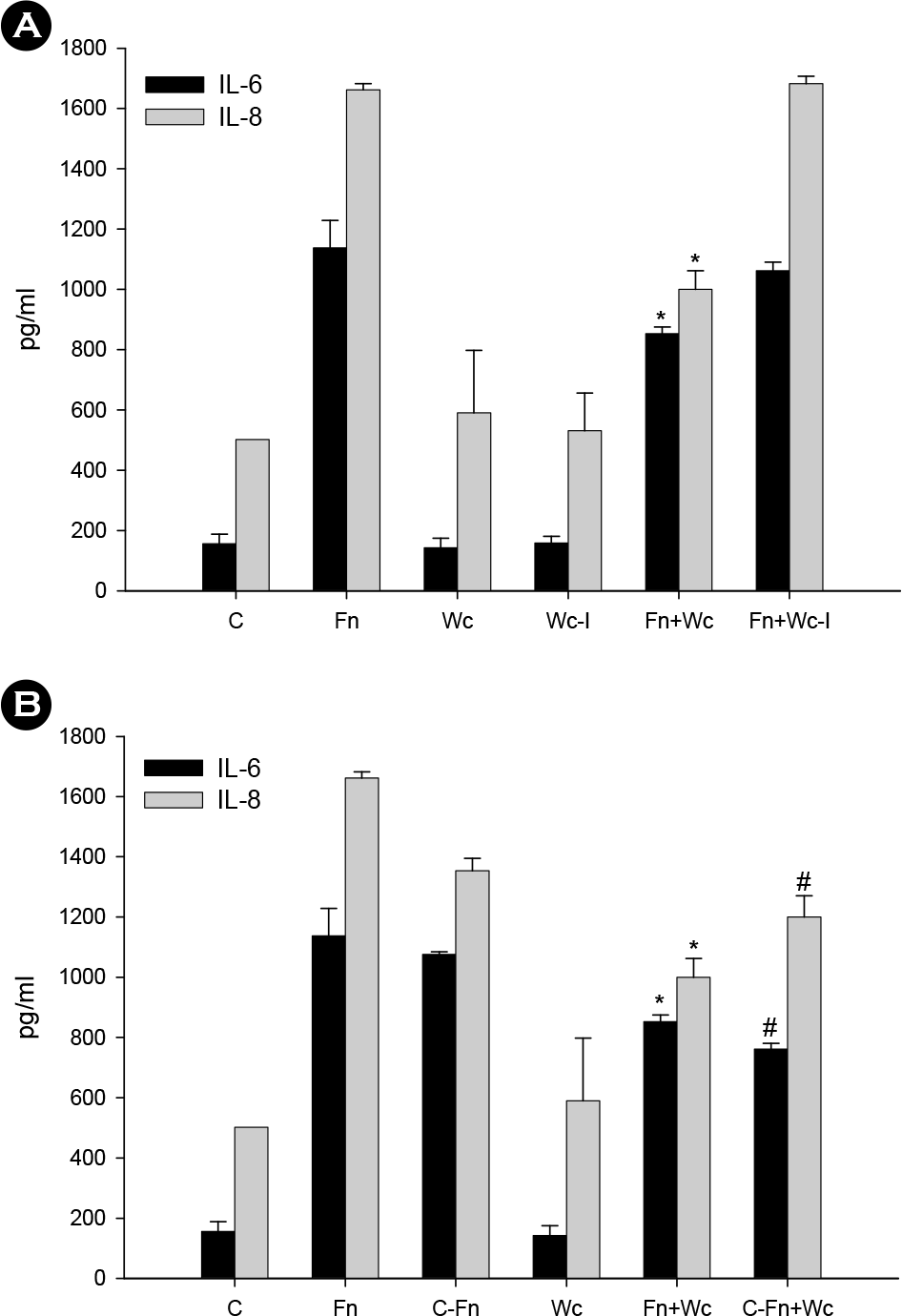 | Figure 3.Effect of the separation of W. cibaria from host cells (A) and coaggregation effect (B) on F. nucleatum-induced IL-6 and IL-8 production in KB cells. IL-6 and IL-8 protein were assayed by ELISA. The data is expressed with the mean ± SD of a representative experiment performed in triplicate. C, control cells without stimulation; Fn, F. nucleatum; Wc, W. cibaria; Wc-I, W. cibaria separated from KB cells through a tissue insert; Fn+Wc, F. nucleatum with W. cibaria; Fn+Wc-I, F. nucleatum with W. cibaria separated from KB cells through a tissue insert; C-Fn, L-canavanine pre-treated F. nucleatum; C-Fn+Wc, L-canavanine pre-treated F. nucleatum with W. cibaria. ∗p < 0.05, compared with F. nucleatum alone; #p < 0.05, compared with L-canavanine pre-treated F. nucleatum alone. |
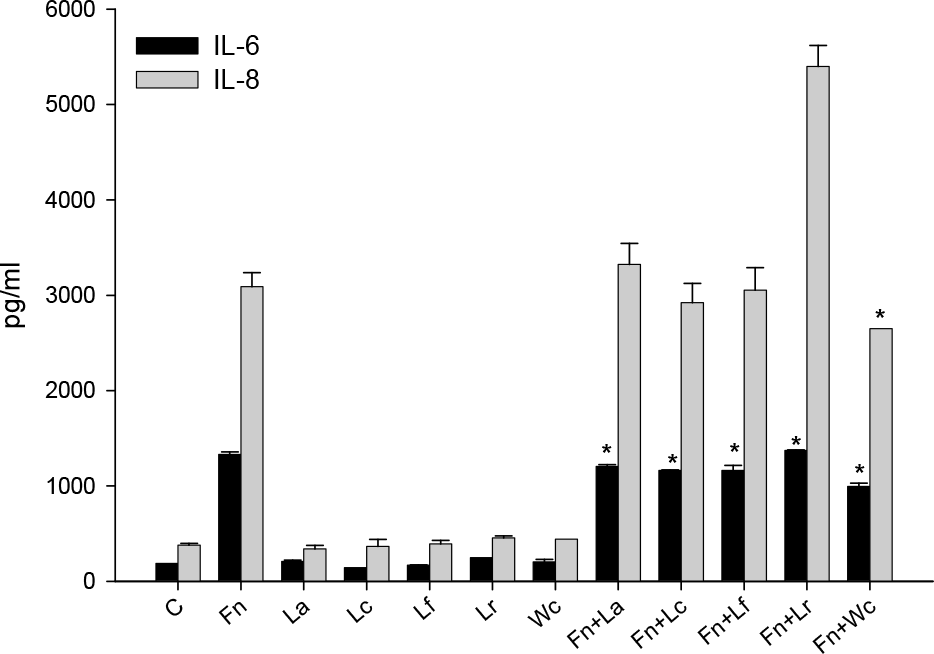 | Figure 4.Effects of various lactobacilli on F. nucleatum-induced IL-6 and IL-8 production in KB cells. W. cibaria and four different Lactobacillus species were used, either alone or in combination with F. nucleatum, to infect the KB cells. IL-6 and IL-8 protein were assayed by ELISA. The data is expressed with the mean ± SD of a representative experiment performed in triplicate. C, control cells without stimulation; Fn, F. nucleatum; La, L. acidophilus; Lc, L. casei; Lf, L. fermentum; Lr, L. reuteri; Wc, W. cibaria; Fn+La, F. nucleatum with L. acidophilus; Fn+Lc, F. nucleatum with L. casei; Fn+Lf, F. nucleatum with L. fermentum; Fn+Lr, F. nucleatum with L. reuteri; Fn+Wc, F. nucleatum with W. cibaria. ∗p < 0.05, compared with F. nucleatum alone. |
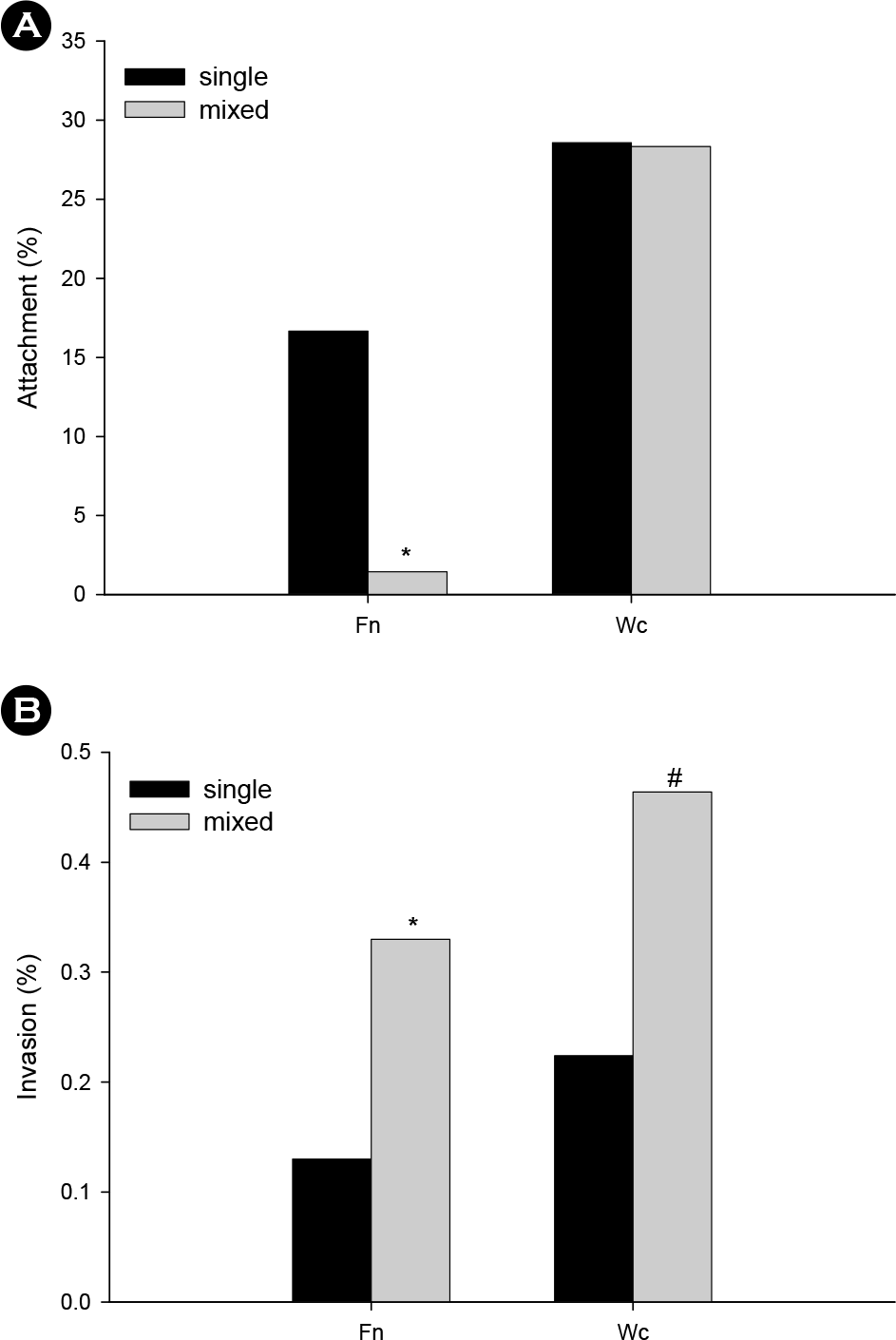 | Figure 5.Effect of W. cibaria on the attachment (A) and invasion (B) of F. nucleatum to KB cells. KB cells were pre-treated with W. cibaria for 1 h and stimulated with F. nucleatum, as described in Materials and Methods. The levels of attachment and invasion is expressed as a percentage of bacteria retrieved following cell lysis relative to the total number of bacteria initially added. The data is expressed as the mean of a representative experiment performed in triplicate. Fn, F. nucleatum; Wc, W. cibaria. ∗p < 0.05, compared with F. nucleatum alone; #p < 0.05, compared with W. cibaria alone. |
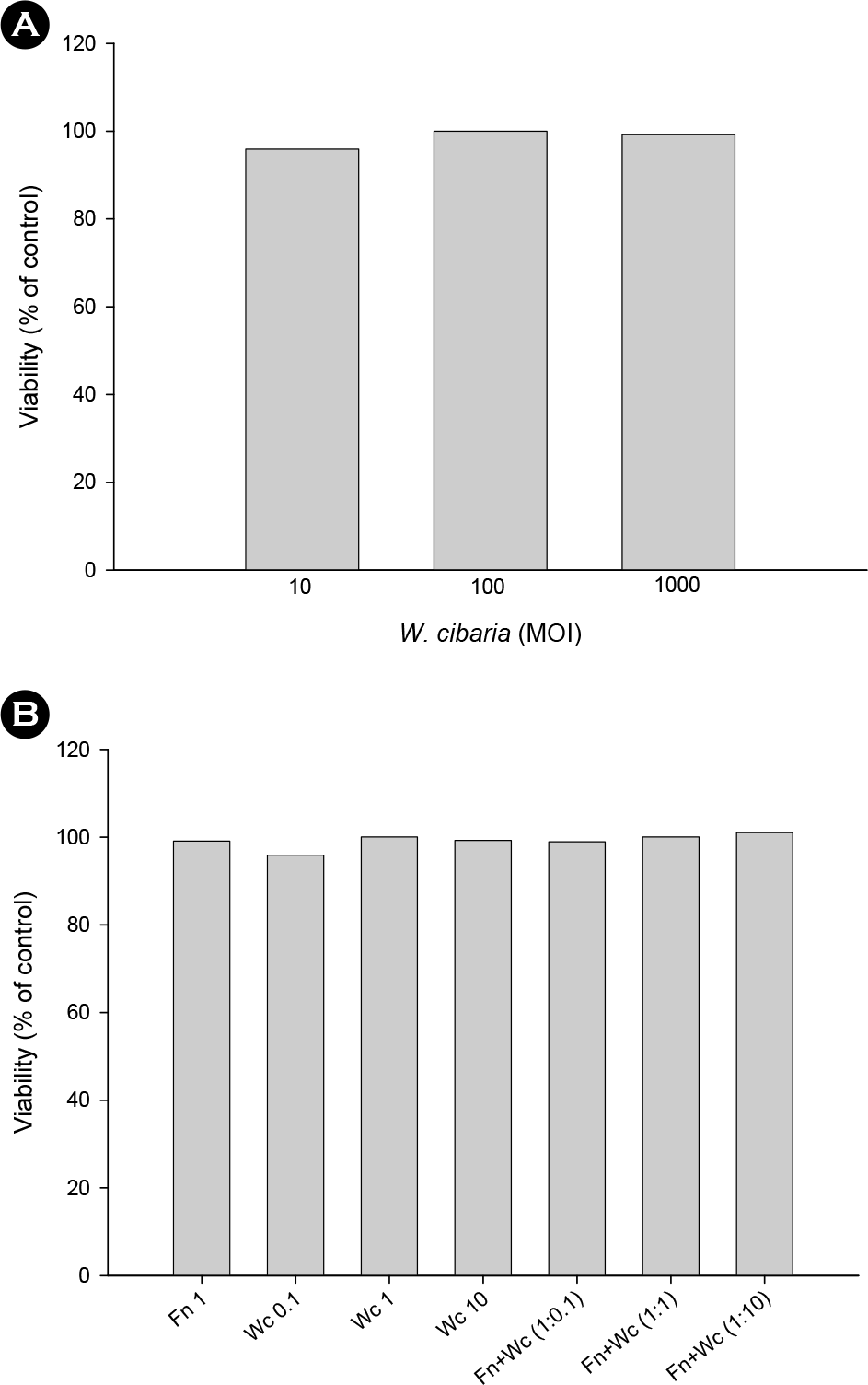 | Figure 6.Cytotoxicity of W. cibaria on KB cells. (A) KB cells were stimulated with W. cibaria at various concentrations (MOI of 10, 100, 1,000) for 24 h. (B) W. cibaria and F. nucleatum were co-cultured with various concentrations (0.1:1, 1:1, 10:1). Untreated cells were used as a control. The cell viability was assessed using a MTT assay. The data is expressed at the mean of a representative experiment performed in triplicate. Fn 1, F. nucleatum was used at a MOI of 100; Wc 0.1, W. cibaria was used at a MOI of 10; Wc 1, W. cibaria was used at a MOI of 100; Wc 10, W. cibaria was used at a MOI of 1000; Fn+Wc (1:0.1), 0.1 W. cibaria per F. nucleatum; Fn+Wc (1:1), 1 W. cibaria per F. nucleatum; Fn+Wc (1:10), 10 W. cibaria per F. nucleatum. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download