Abstract
Lactic acid bacteria (LAB), including L. plantarum isolated from Kimchi, are beneficial and safe microorganisms that improve disturbances of the indigenous microflora and the host's immune system. The adhesion abilities of Kimchi-derived L. plantarum PM008 and yogurt-derived L. casei were measured in vitro and in vivo. When L. plantarum or L. casei was incubated with Caco-2 cells, these Lactobacillus strains were potently attached. When these strains were orally administered to mice, the LABs were attached on the large intestine of mice. The attachment of L. plantarum on murine intestine or Caco-2 intestinal epithelial cell lines was more potent than that of L. casei, although numbers of LAB between their feces were not different. Treatment with either L. plantarum or L. casei for 14 days suppressed fecal β-glucuronidase activity, although treatment for one day did not affect it. L. plantarum showed more potent inhibition than L. casei. In addition, L. plantarum and L. casei were stable to artificial gastric and intestinal juice. L. plantarum was more stable than L. casei. Based on these findings, the survival and adhesion effects of orally administered LAB strains in the intestine may increase numbers of LAB in intestine and express their biological activities.
Go to : 
REFERENCES
2). Simon GL., Gorbach SL. Intestinal flora in health and disease. Gastroenterology. 1984. 86:174–93.

3). Hattori M., Taylor TD. The human intestinal micro-biome: a new frontier of human biology. DNA Res. 2009. 16:1–12.

4). Chung KT., Fulk GE., Slein MW. Tryptophanase of fecal flora as a possible factor in the etiology of colon cancer. J Natl Cancer Inst. 1975. 54:1073–8.
5). Ganguly NK., Kingham JG., Lloyd B., Lloyd RS., Price CP., Triger DR, et al. Acid hydrolases in monocytes from patients with inflammatory bowel disease, chronic liver disease, and rheumatoid arthritis. Lancet. 1978. 1:1073–5.

6). Rhodes JM., Gallimore R., Elias E., Allan RN., Kennedy JF. Faecal mucus degrading glycosidases in ulcerative colitis and Crohn's disease. Gut. 1985. 26:761–5.

7). Reddy BS., Weisburger JH., Wynder EL. Fecal bacterial beta-glucuronidase: control by diet. Science. 1974. 183:416–7.
8). Collins MD., Gibson GR. Probiotics, prebiotics, and synbiotics: approaches for modulating the microbial ecology of the gut. Am J Clin Nutr. 1999. 69:1052S–1057S.

9). Campieri M., Gionchetti P. Probiotics in inflammatory bowel disease: New insight to pathogenesis or a possible therapeutic alternative? Gastroenterology. 1999. 116:1246–9.

10). Tabuchi M., Ozaki M., Tamura A., Yamada N., Ishida T., Hosoda M, et al. Antidiabetic effect of Lactobacillus GG in streptozotocin-induced diabetic rats. Biosci Biotechnol Biochem. 2003. 67:1421–4.
11). Taranto MP., Medici M., Perdigon G., Ruiz Holgado AP., Valdez GF. Evidence for hypocholesterolemic effect of Lactobacillus reuteri in hypercholesterolemic mice. J Dairy Sci. 1998. 81:2336–40.
12). Perdigon G., de Jorrat WEB., de Petrino SF., de Budeguer MV. Effect of oral administration of Lactobacillus casei on various biological functions of the host. Food Agric Immunol. 1991. 3:93–102.
13). Daniel C., Poiret S., Goudercourt D., Dennin V., Leyer G., Pot B. Selecting lactic acid bacteria for their safety and functionality by use of a mouse colitis model. Appl Environ Microbiol. 2006. 72:5799–805.

14). Han W., Mercenier A., Ait-Belgnaoui A., Pavan S., Lamine F., van Swam II, et al. Improvement of an experimental colitis in rats by lactic acid bacteria producing super-oxide dismutase. Inflamm Bowel Dis. 2006. 12:1044–52.

15). Peran L., Sierra S., Comalada M., Lara-Villoslada F., Bailon E., Nieto A, et al. A comparative study of the preventative effects exerted by two probiotics, Lactobacillus reuteri and Lactobacillus fermentum, in the trinitrobenzenesulfonic acid model of rat colitis. Br J Nutr. 2007. 97:96–103.
16). Mare L., Wolfaardt GM., Dicks LM. Adhesion of Lactobacillus plantarum 423 and Lactobacillus salivarius 241 to the intestinal tract of piglets, as recorded with fluorescent in situ hybridization (FISH), and production of plantaricin 423 by cells colonized to the ileum. J Appl Micribiol. 2006. 100:838–45.
17). Sheiness D., Dix K., Watanabe S., Hillier SL. High levels of Gardnerella vaginalis detected with an oligonuceotide probe combined with elevated pH as a diagnostic indicator of bacterial vaginosis. J Clin Microbiol. 1992. 30:642–8.
18). Amann RI., Binder BJ., Olson RJ., Chisholm SW., Devereux R., Stahl DA. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol. 1990. 56:1919–25.

19). Cho J., Lee D., Yang C., Jeon J., Kim J., Han H. Microbial population dynamics of kimchi, a fermented cabbage product. FEMS Microbiol Lett. 2006. 257:262–7.

20). Lee J., Hwang KT., Heo MS., Lee JH., Park KY. Resistance of Lactobacillus plantarum KCTC 3099 from Kimchi to oxidative stress. J Med Food. 2005. 8:299–304.
21). Cho YR., Chang JY., Chang HC. Production of gamma-aminobutyric acid (GABA) by Lactobacillus buchneri isolated from kimchi and its neuroprotective effect on neuronal cells. J Microbiol Biotechnol. 2007. 17:104–9.
22). Parente E., Ciocia F., Ricciardi A., Zotta T., Felis GE., Torriani S. Diversity of stress tolerance in Lactobacillus plantarum, Lactobacillus pentosus and Lactobacillus paraplantarum: A multivariate screening study. Int J Food Microbiol. 2010. 144:270–9.
23). Arena ME., Saguir FM., Manca de Nadra MC. Arginine dihydrolase pathway in Lactobacillus plantarum from orange. Int J Food Microbiol. 1999. 47:203–9.
24). Siragusa S., De Angelis M., Di Cagno R., Rizzello CG., Coda R., Gobbetti M. Synthesis of gamma-amminobutyric acid by lactic acid bacteria isolated from a variety of Italian cheeses. Appl Environ Microbiol. 2007. 73:7283–90.
25). Maragkoudakis PA., Chingwaru W., Gradisnik L., Tsakalidou E., Cencic A. Lactic acid bacteria efficiently protect human and animal intestinal epithelial and immune cells from enteric virus infection. Int J Food Microbiol. 2010. ;141 Suppl. 1:S91–7.

26). Park HY., Bae EA., Han MJ., Choi EC., Kim DH. Inhibitory effects of Bifidobacterium spp. isolated from a healthy Korean on harmful enzymes of human intestinal microflora. Arch Pharm Res. 1998. 21:54–61.
Go to : 
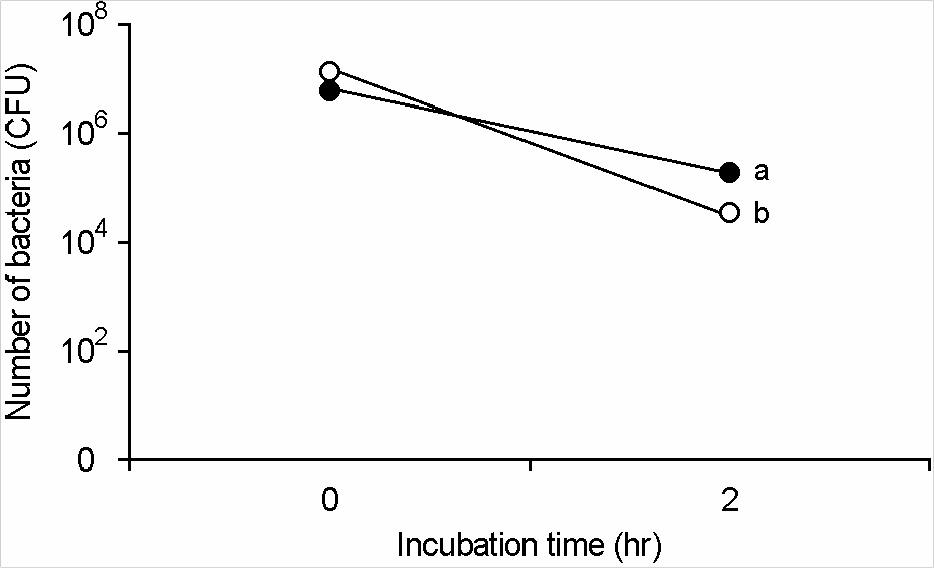 | Figure 1.Adhesion ability of lactic acid bacteria to Caco-2 cells. Lactic acid bacteria (closed circle, 1 × 107 L. plantarum per well; open circle, 1.0 × 107 L. casei per well) were incubated with Caco-2 cells for 2 h, washed with PBS and then inoculated in MRS agar plate. |
 | Figure 2.Number of intestinal bacteria attached in colon membrane in Lactobacillus plantarum (A) or Lactobacillus casei (B)-treated mice by a fluorescent hybridization method. Lactic acid bacteria were orally administered once a day for one (a) or 14 days (b): LP1, treated with 1.0 × 109 L. plantarum per mouse for one day; LP14, 1.0 × 109 treated with L. plantarum per mouse for 14 days; LC1, 1.0 × 109 L. casei per mouse for one day; LC14, 1.0 × 109 treated with L. casei per mouse for 14 days). All values are mean ± SD. (n = 6). ∗significantly different compared to control group. |
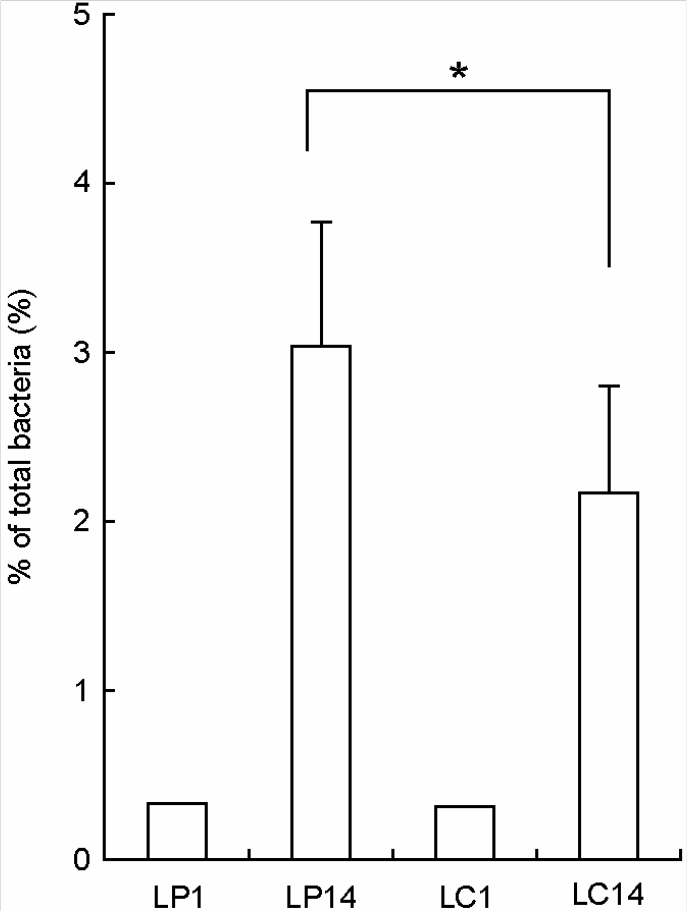 | Figure 3.Number of intestinal bacteria attached in colon membrane in Lactobacillus plantarum (LP) or Lactobacillus casei (LC)-treated mice by a slot blot hybridization method. Lactic acid bacteria were orally administered once a day for one or 14 days: LP1, treated with 1.0 × 109 L. plantarum per mouse for one day; LP14, 1.0 × 109 treated with L. plantarum per mouse for 14 days; LC1, 1.0 × 109 L. casei per mouse for one day; LC14, 1.0 × 109 treated with L. casei per mouse for 14 days). All values are mean ± SD. (n=6). ∗significantly different compared to control group. |
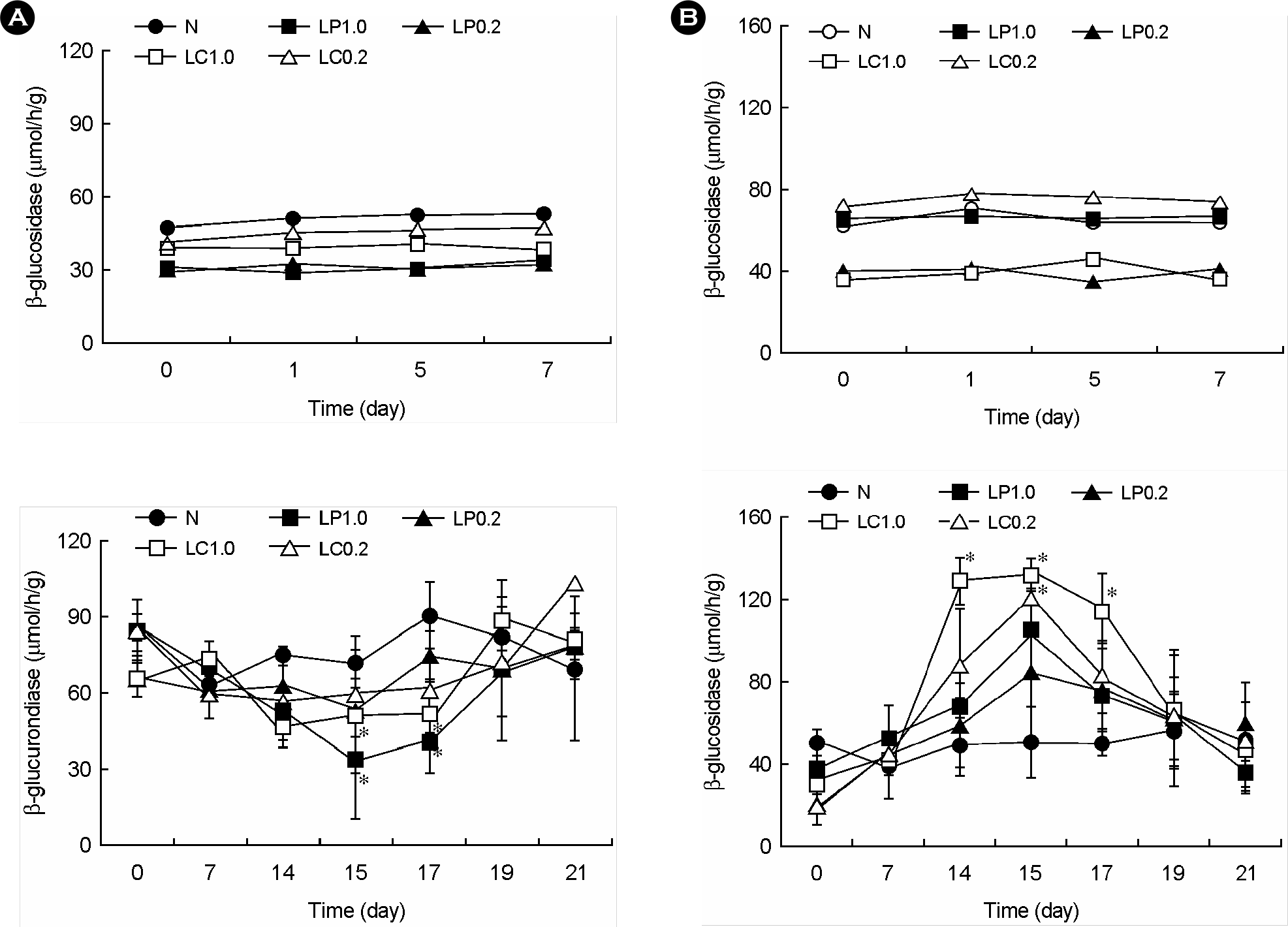 | Figure 4.Effect of lactic acid bacteria on fecal bacterial. β-glucosidase (A) and β-glucuronidase activities (B) in mice. Lactic acid bacteria (LP0.2, 0.2 × 109 L. plantarum per mouse; LP1.0, 1.0 × 109 L. plantarum per mouse; LC0.2, 0.2 × 109 L. casei per mouse; LC1.0, 1 × 109 L. casei per mouse) were orally administered once a day for one (upper) or 14 days (bottom). All values are mean ± SD. (n=6). ∗significantly different compared to control group. |
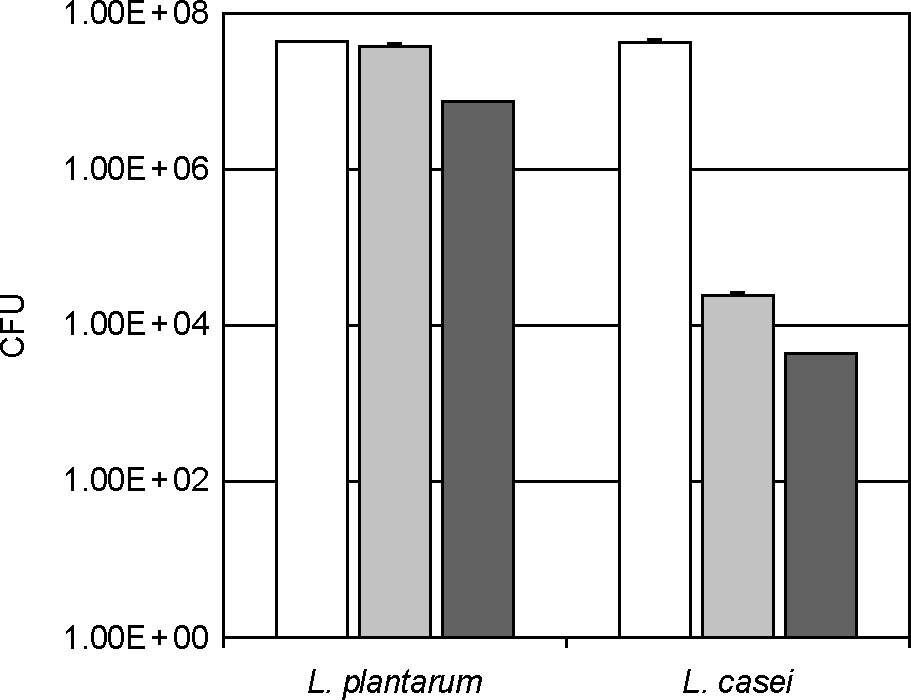 | Figure 5.Stability of lactic acid bacteria against artificial gastric juice and intestinal juice. Lactic acid bacteria (L. plantarum or L. casei) were incubated in saline (white bar), artificial gastric [gray bar, pepsin (1,000 units)-contained MRS broth adjusted at pH 2.5 with 5 N HCl] or intestinal juice [black bar, GAM broth containing 0.1% mucin, 0.04% pancreatin, 0.2% bile, 0.85% NaCl, and 0.04% trypsin (pH 6.8)] for 3 h at 37°C and number of survival LAB were determined by plating serial dilutions (in PBS, pH 7.2) on MRS agar followed by incubation at 37°C for 48 h. All values are mean ± SD. (n=6). ∗significantly different compared to saline control group. |
Table 1.
Colony forming unit (CFU) of fecal bacteria grown in MRS and BL agar plates in mice orally treated with lactic acid bacteria
Lactic acid bacteria (L. plantarum or L. casei) were orally administered once a day for one or 14 days. Numbers of intestinal bacteria were counted by plating serial dilutions (in diluted anaerobic broth, pH 7.2) on MRS and BL agars followed by anaerobic incubation at 37°C for 48 h. All values are mean (n=3).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download