Abstract
Burkholderia pseudomallei is a gram-negative opportunistic intracellular pathogen that causes an acute and fatal septicemic melioidosis in humans. The organism is mainly found in Southeastern Asia and Northern Australia. Recently, we encountered a case of melioidosis in a Korean patient and performed the laboratory diagnosis of melioidosis. As a result, a gram negative bacterium was isolated from a melioidosis patient, and it was identified as B. pseudomallei on DNA sequencing of 16S ribosomal RNA with 99.9% homology and biochemical examination of VITEK gram-negative identification card. Also, DNA from cultured bacteria was tested in multiplex PCR, a 245 bp fragment amplified from the metalloprotease gene and a fragment of variable size ranging from 400~700 bp resulting from amplification of the 10 bp repetitive element for B. pseudomallei were confirmed after electrophoresis. The bacterium was sensitive to ceftazidime, imipenem and meropenem but resistant to ticarcillin. So far, there are no domestic cases of melioidosis in Korea, however, due to the increase in international travelers, the incidence of melioidosis is likely to increase. We report a recent case of melioidosis in a Korean patient.
Go to : 
REFERENCES
1). Currie BJ., Dance DA., Cheng AC. The global distribution of Burkholderia pseudomallei and melioidosis: an update. Trans R Soc Trop Med Hyg. 2008. 102:S1–4.
2). Cheng AC., Currie BJ. Melioidosis: epidemiology, patho-physiology, and management. Clin Microbiol Rev. 2005. 18:383–416.

3). Seok HJ., Kim JI., Lee JH., Choo EJ., Kwak YG., Jang S, et al. A case of septic pneumonia due to Burkholderia pseudomallei. Infect Chemother. 2004. 36:114–7.
4). Lee SW., Yi J., Joo SI., Kang YA., Yoon YS., Yim JJ, et al. A case of melioidosis presenting as migrating pulmonary infiltration: the first case in Korea. J Korean Med Sci. 2005. 20:139–42.

5). Son JY., Kwon KT., Choi EJ., Park JP., Song YD., Lee JC, et al. Fatal melioidosis in a tourist returning from Cambodia. Korean J Med. 2009. 77:246–50.
6). Lee HM., Choi SH., Chung JW., Ahn J., Cho AR., Lee MK, et al. A case of disseminated melioidosis in a migrant worker from Thailand. Korean J Lab Med. 2009. 29:140–4.

7). Current status and laboratory diagnosis of melioidosis, Korea Centers for Disease Control and Prevention. Public Health Wkly Rep. 2010. 3:276–9.
8). Ashdown LR. Identification of Pseudomonas pseudomallei in the clinical laboratory. J Clin Pathol. 1979. 32:500–4.
9). Lee MA., Wang D., Yap EH. Detection and differentiation of Burkholderia pseudomallei, Burkholderia mallei and Burkholderia thailandensis by multiplex PCR. FEMS Immunol Med Microbiol. 2005. 43:413–7.
10). Epidemiologic investigation for a melioidosis mortality case, Korea Centers for Disease Control and Prevention. Public Health Wkly Rep. 2010. 3:180.
11). Gan YH. Interaction between Burkholderia pseudomallei and the host immune response: sleeping with the enemy? J Infect Dis. 2005. 192:1845–50.
Go to : 
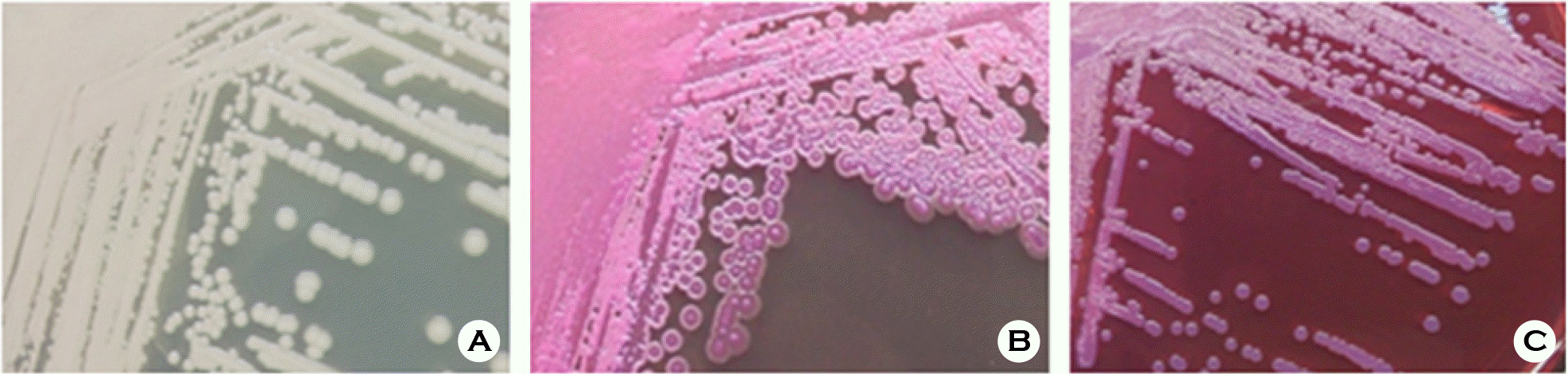 | Figure 1.Colonial growth morphology of Burkholderia pseudomallei. (A) Colonies on BHI agar after 48 h culture; (B) Colonies on MacConkey agar after 48 h culture; (C) Colonies on Ashdown's medium after 48 h culture. |
 | Figure 2.Comparison of the 16S rRNA sequences from B. pseudomallei (GenBank accession no. CP000572) and B. pseudomallei isolated from a melioidosis patient. Bold letters show the positions of the 3 nucleotide dissimilarities between B. pseudomallei and B. pseudomallei isolated from a melioidosis patient. |
 | Figure 3.The phylogenetic tree based on the 16S rRNA gene sequences for B. pseudomallei isolate and other Burkholderia. The phylogenetic tree was generated using Neighbor-joining method based on DNA sequence of partial 16S rDNA gene. Bootstrap values of above 50% from a sample of 1,000 replicate are shown on each branch. |
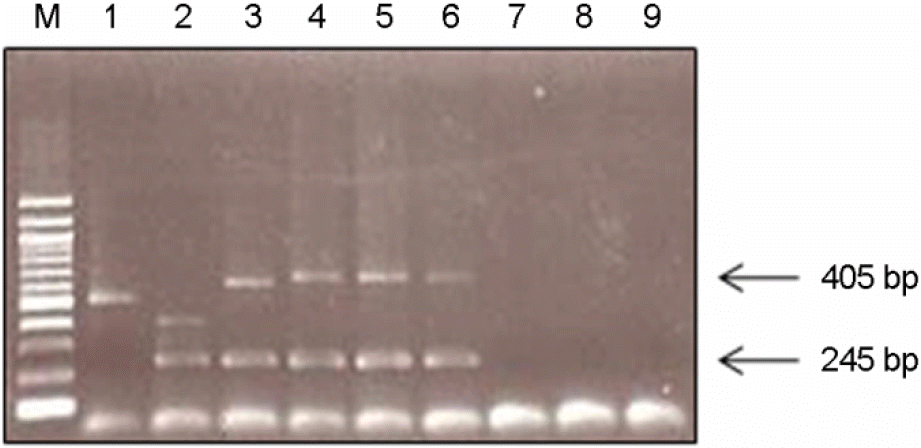 | Figure 4.Multiplex PCR analysis of B. mallei, B. thailandensis and B. pseudomallei isolates. Lane 1. B. mallei; lane 2. B. thailandensis; lane 3. B. pseudomallei; lane 4. Genomic DNA isolated directly from blood; lane 5. B. pseudomallei cultured from blood; lane 6. B. pseudomallei cultured from sputum; lane 7. B. cepacia; lane 8. P. aeruginosa; lane 9. E.coli. |
Table 1.
Primers used for multiplex PCR
Table 2.
Antimicrobial susceptibility patterns of B. pseudomallei
| Antimicrobial agents | Breakpoint for resistance | MIC (μg/ml) | Resistance |
|---|---|---|---|
| Ticarcillin | >64b | ≥128 | R |
| Ticarcillin/clavulanate | ≤16/2b | ≤8 | S |
| Ampicillin/sulbactam | ND | 4 | ND |
| Piperacillin | ≤16b | ≤4 | S |
| Penicillin/tazobactam | ≤16/4b | ≤4 | S |
| Cefotaxime | ND | 8 | ND |
| Ceftazidime | ≤4b | ≤1 | S |
| Cefepime | >8b | 8 | I |
| Aztreonam | >16a | 16 | I |
| Imipenem | ≤2b | ≤1 | S |
| Meropenem | ≤2b | 1 | S |
| Amikacin | ≤8b | ≤2 | S |
| Gentamicin | ≤4b | ≤1 | S |
| Tobramycin | ≤4b | ≤1 | S |
| Ciprofloxacin | ≤1b | 1 | I |
| Levofloxacin | ≤1b | 1 | I |
| Minocycline | ≤4a | ≤1 | S |
| Trimethoprim/sulfamethoxazole | ≤4/76b | ≤20 | S |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download