Abstract
Porcine reproductive and respiratory syndrome virus (PRRSV) is the causative agent of reproductive failure and respiratory disorders in pigs. The viral genome consists of eight overlapping open reading frames (ORFs). ORF5 encodes one of the major glycoproteins and is known as an immunologically important structural protein associated with virus neutralization. The ORF5 gene of the Korean PRRSV isolate, CNV-1, was amplified by reverse transcription-polymerase chain reaction (RT-PCR), cloned and sequenced. The nucleotide and amino acid sequences of CNV-1 ORF5 shared 91% and 83% identity, respectively, with the American isolate (VR2332 strain) and 57% and 49% identity with the European isolate. For the expression and easy purification of ORF5, the cDNA containing the complete ORF5 sequence fused in-frame with sequence encoding glutathione S-transferase (GST) was cloned into a baculovirus transfer vector and transfected into Sf9 cells. The GST-ORF5 fusion protein produced in Sf9 cells was detected by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting. Sequencing results confirmed that the recombinant baculovirus from Sf9 cells contains the complete ORF5 gene. Further studies in this direction will address whether ORF5 can be a good candidate for a subunit vaccine against PRRSV in Korea.
Go to : 
REFERENCES
1). Albina E. Epidemiology of porcine reproductive and respiratory syndrome (PRRS): an overview. Vet Microbiol. 1997. 55:309–16.

2). Wootton SK., Nelson EA., Yoo D. Antigenic structure of the nucleocapsid protein of porcine reproductive and respiratory syndrome virus. Clin Diagn Lab Immunol. 1998. 5:773–9.

3). Lunney JK., Benfield DA., Rowland RR. Porcine reproductive and respiratory syndrome virus: an update on an emerging and re-emerging viral disease of swine. Virus Res. 2010. 154:1–6.

4). Cavanagh D. Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch Virol. 1997. 142:629–33.
5). Conzelmann KK., Visser N., van Woensel P., Thiel HJ. Molecular characterization of porcine reproductive and respiratory syndrome virus, a member of the arterivirus group. Virology. 1993. 193:329–39.

6). Meulenberg JJ., Hulst MM., de Meijer EJ., Moonen PL., den Besten A., de Kluyver EP, et al. Lelystad virus, the causative agent of porcine epidemic abortion and respiratory syndrome (PEARS), is related to LDV and EAV. Virology. 1993. 192:62–72.

7). Cha SH., Choi EJ., Park JH., Yoon SR., Song JY., Kwon JH, et al. Molecular characterization of recent Korean porcine reproductive and respiratory syndrome (PRRS) viruses and comparison to other Asian PRRS viruses. Vet Microbiol. 2006. 31:248–57.

8). Indik S., Valícek L., Klein D., Klánová J. Variations in the major envelope glycoprotein GP5 of Czech strains of porcine reproductive and respiratory syndrome virus. J Gen Virol. 2000. 81:2497–502.

9). Kiss I., Sámi L., Kecskeméti S., Hanada K. Genetic variation of the prevailing porcine respiratory and reproductive syndrome viruses occurring on a pig farm upon vaccination. Arch Virol. 2006. 151:2269–76.

10). Pirzadeh B., Dea S. Immune response in pigs vaccinated with plasmid DNA encoding ORF5 of porcine reproductive and respiratory syndrome virus. J Gen Virol. 1998. 79:989–99.

11). Goldberg TL., Hahn EC., Weigel RM., Scherba G. Genetic, geographical and temporal variation of porcine reproductive and respiratory syndrome virus in Illinois. J Gen Virol. 2000. 81:171–9.

12). Kapur V., Elam MR., Pawlovich TM., Murtaugh MP. Genetic variation in porcine reproductive and respiratory syndrome virus isolates in the midwestern United States. J Gen Virol. 1996. 77:1271–6.

13). Okuda Y., Kuroda M., Ono M., Chikata S., Shibata I. Efficacy of vaccination with porcine reproductive and respiratory syndrome virus following challenges with field isolates in Japan. J Vet Med Sci. 2008. 70:1017–25.

14). Pirzadeh B., Gagnon CA., Dea S. Genomic and antigenic variations of porcine reproductive and respiratory syndrome virus major envelope GP5 glycoprotein. Can J Vet Res. 1998. 62:170–7.
15). Mengeling WL., Lager KM., Vorwald AC. Safety and efficacy of vaccination of pregnant gilts against porcine reproductive and respiratory syndrome. Am J Vet Res. 1999. 60:796–801.
16). Cano JP., Dee SA., Murtaugh MP., Pijoan C. Impact of modified-live porcine reproductive and respiratory syndrome virus vaccine intervention on a population of pigs infected with a heterologous isolate. Vaccine. 2007. 25:4382–91.
17). Nielsen HS., Oleksiewicz MB., Forsberg R., Stadejek T., B⊘tner A., Storgaard T. Reversion of a live porcine reproductive and respiratory syndrome virus vaccine investigated by parallel mutations. J Gen Virol. 2001. 82:1263–72.

18). Thanawongnuwech R., Halbur PG., Ackermann MR., Thacker EL., Royer RL. Effects of low (modified-live virus vaccine) and high (VR-2385)-virulence strains of porcine reproductive and respiratory syndrome virus on pulmonary clearance of copper particles in pigs. Vet Pathol. 1998. 35:398–406.

19). Wesley RD., Mengeling WL., Lager KM., Clouser DF., Landgraf JG., Frey ML. Differentiation of a porcine reproductive and respiratory syndrome virus vaccine strain from North American field strains by restriction fragment length polymorphism analysis of ORF 5. J Vet Diagn Invest. 1998. 10:140–4.

20). Cano JP., Dee SA., Murtaugh MP., Pijoan C. Impact of a modified-live porcine reproductive and respiratory syndrome virus vaccine intervention on a population of pigs infected with a heterologous isolate. Vaccine. 2007. 25:4382–91.

21). Tacket CO., Losonsky G., Lubeck MD., Davis AR., Mizutani S., Horwith G, et al. Initial safety and immunogenicity studies of an oral recombinant adenohepatitis B vaccine. Vaccine. 1992. 10:673–6.

22). Kwang J., Zuckermann F., Ross G., Yang S., Osorio F., Liu W, et al. Antibody and cellular immune responses of swine following immunisation with plasmid DNA encoding the PRRS virus ORF's 4, 5, 6 and 7. Res Vet Sci. 1999. 67:199–201.

23). Plana Duran J., Climent I., Sarraseca J., Urniza A., Cortés E., Vela C, et al. Baculovirus expression of proteins of porcine reproductive and respiratory syndrome virus strain Olot/91. Involvement of ORF3 and ORF5 proteins in protection. Virus Genes. 1997. 14:19–29.
24). Koo HN., Oh JM., Lee JK., Choi JY., Lee KS., Roh JY, et al. Molecular characterization of ORFs 2 to 7 of Korean porcine reproductive and respiratory syndrome virus (CA) and its protein expression by recombinant baculo-viruses. J Microbiol. 2008. 46:709–19.

25). Kreutz LC., Mengeling WL. Baculovirus expression and immunological detection of the major structural proteins of porcine reproductive and respiratory syndrome virus. Vet Microbiol. 1997. 59:1–13.

26). Jung HS., Hwang IW., Kim SM., Kim CJ., Shin KS., Kim HS. Expression of open reading frame 5 protein of porcine reproductive and respiratory syndrome virus using semliki forest virus expression system. J Vet Sci. 2002. 3:13–8.

27). Li Y., Wang X., Jiang P., Chen W., Wang X. Genetic analysis of two porcine reproductive and respiratory syndrome viruses with different virulence isolated in China. Arch Virol. 2008. 153:1877–84.

28). Nam E., Park CK., Kim SH., Joo YS., Yeo SG., Lee C. Complete genomic characterization of a European type 1 porcine reproductive and respiratory syndrome virus isolate in Korea. Arch Virol. 2009. 154:629–38.

29). Calvin NM., Hanawalt PC. High-efficiency transformation of bacterial cells by electroporation. J Bacteriol. 1988. 170:2796–801.

30). Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970. 227:680–5.

31). Le CT., Gray GC., Poddar SK. A modified rapid method of nucleic acid isolation from suspension of matured virus: applied in restriction analysis of DNA from an adenovirus prototype strain and a patient isolate. J Med Microbiol. 2001. 50:571–4.

32). Balasuriya UB., Timoney PJ., McCollum WH., MacLachlan NJ. Phylogenetic analysis of open reading frame 5 of field isolates of equine arteritis virus and identification of conserved and nonconserved regions in the GL envelope glycoprotein. Virology. 1995. 214:690–7.
33). Fang Y., Schneider P., Zhang WP., Faaberg KS., Nelson EA., Rowland RR. Diversity and evolution of a newly emerged North American Type 1 porcine arterivirus: analysis of isolates collected between 1999 and 2004. Arch Virol. 2007. 152:1009–17.

34). Meng XJ., Paul PS., Halbur PG., Morozov I. Sequence comparison of open reading frames 2 to 5 of low and high virulence United States isolates of porcine reproductive and respiratory syndrome virus. J Gen Virol. 1995. 76:3181–8.

35). Murtaugh MP., Elam MR., Kakach LT. Comparison of the structural protein coding sequences of the VR-2332 and Lelystad virus strains of the PRRS virus. Arch Virol. 1995. 140:1451–60.

Go to : 
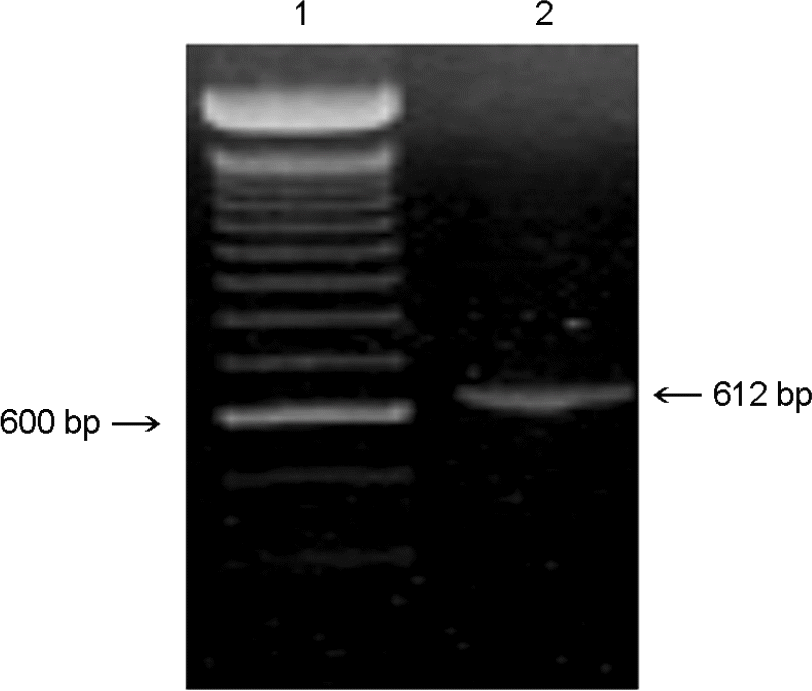 | Figure 1.Amplification of CNV-1 ORF5. Lanes: 1, 100 bp ladder; 2, CNV-1. The numbers on both sides indicate the size in bp. |
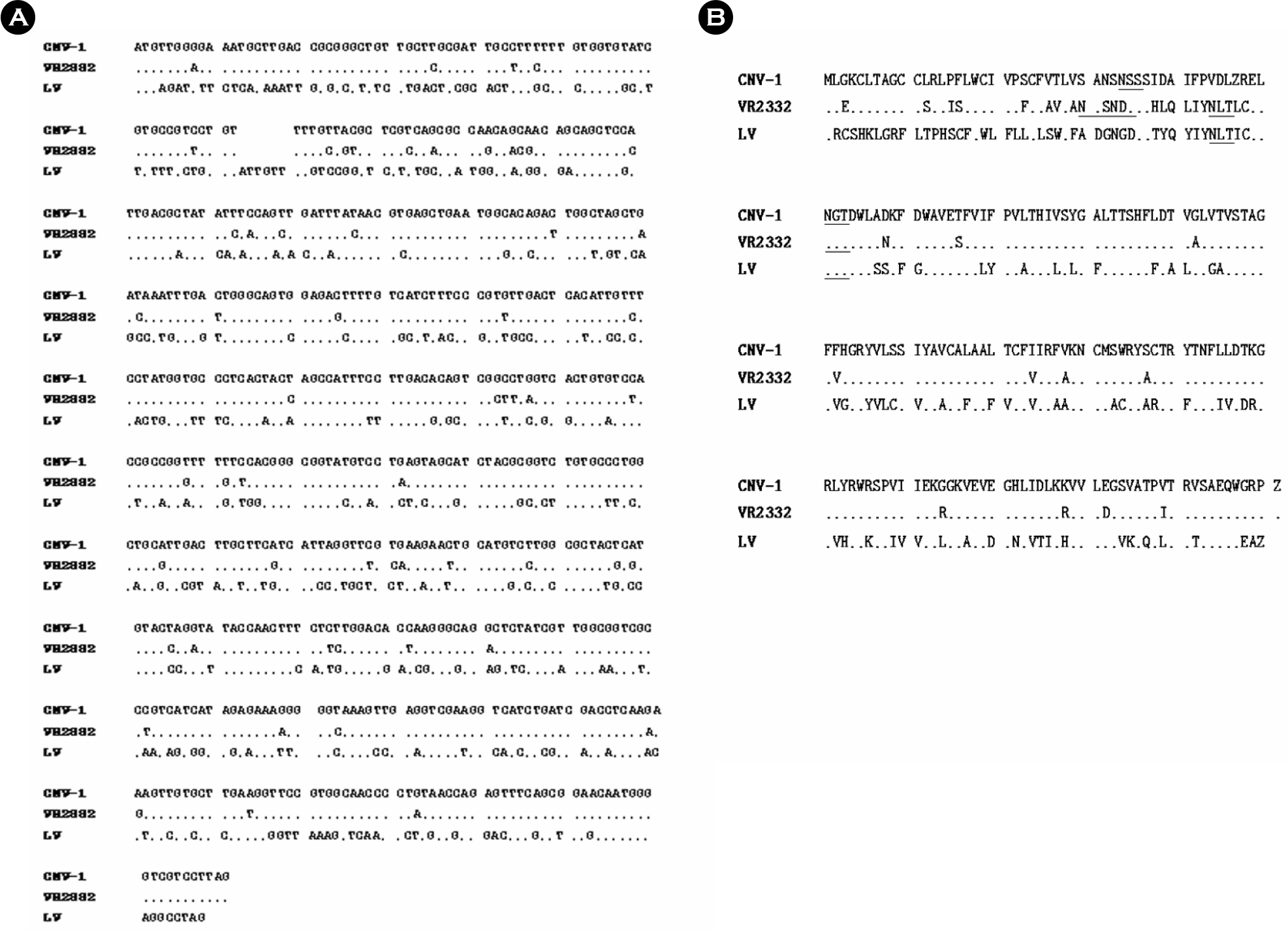 | Figure 2.Nucleotide sequences (A) and amino acid sequences (B) of CNV-1 ORF5 compared with those of VR2332 (accession number U87392) and LV (accession number NC_002533). Underlines indicate putative N-glycosylatio sites. |
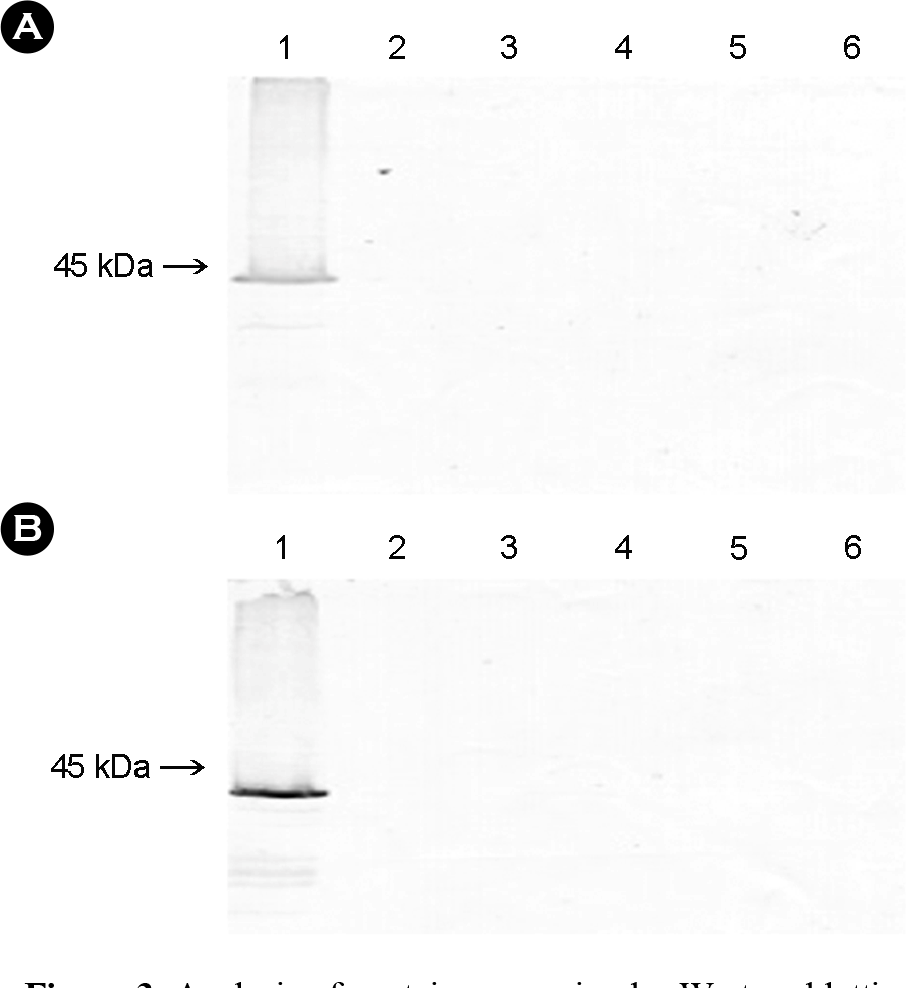 | Figure 3.Analysis of protein expression by Western blottings probed with swine anti-PRRSV serum (A) and mouse anti-GST monospecific antibody (B). The supernatants and the cell pellet were harvested at four days post-infection and were separated by SDS-PAGE, then transferred to nitrocellulose membranes. The proteins were detected with peroxidase-labeled anti-pig IgG (A) and peroxidase-labeled anti-mouse IgG (B). Lanes: 1, lysate of Sf9 cells infected with recombinant baculovirus containing ORF5; 2, lysate of Sf9 cells infected with wild-type AcNPV; 3, lysate of Sf9 cells infected with recombinant Xy1E; 4, supernatant of Sf9 cells infected with recombinant baculovirus containing ORF5; 5, supernatant of Sf9 cells infected with wild-type AcNPV; and 6, lysate of non-infected Sf9 cells. The numbers at the left indicate size markers. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download