Abstract
A heterogenic group of staphylococcal exotoxins, including staphylococcal superantigenic toxins, enterotoxin (SE), toxic shock syndrome toxin-1 (TSST-1), and coagulase are the most important virulence factors of Staphylococcus aureus. We analyzed the prevalence of genes encoding five enterotoxins and TSST-1 in S. aureus isolated from clinical ear discharges. The genes were identified by multiplex PCR and we compared the results to references of coagulase serotypes. In 102 isolates of S. aureus, 44 of them were methicillin-resistant S. aureus (MRSA) and the others were methicillin-susceptible S. aureus (MSSA). Among both types of S. aureus, 33 strains were positive for sea, 2 for seb, 23 for sec, 26 for see, and 26 for tst. Overall, 59 (57.8%) isolates were positive for one or more superantigenic toxin genes. From these, 71.2% (42/59) strains harbored more than one toxin gene in different combinations. The major combinations of genes were sea and see, and sec and tst. The degree of possession of superantigenic toxic genes was similar in both MRSA and MSSA isolates (56.8% vs 58.6%, respectively), yet significant differences in toxin gene profiles and coagulase serotypes between two isolates were detected. All of 13 positive strains for sec and tst were MRSA and belonged to coagulase serotype II. On the other hand, 80.0% of 20 positive strains for sea and see were MSSA with coagulase serotype IV and VII, whereas 20.0% of them were MRSA with coagulase serotype IV. This data indicates that the profile of superantigenic toxin genes correlates to coagulase serotype and methicillin resistance in S. aureus isolates.
Go to : 
REFERENCES
1). Kim YK., Kim JS., Kim HS., Song W., Cho HC., Lee KM. Molecular typing of Staphylococcus aureus isolated from blood on the basis of coagulase gene polymorphism and toxin genes. Korean J Lab Med. 2008. 28:286–92.
2). Eady EA., Cove JH. Staphylococcal resistance revisited: community-acquired methicillin resistant Staphylococcus aureus-an emerging problem for the management of skin and soft tissue infections. Curr Opin Infect Dis. 2003. 16:103–24.
3). Peck KR., Baek JY., Song JH., Ko KS. Comparison of genotypes and enterotoxin genes between Staphylococcus aureus isolates from blood and nasal colonizers in a Korean hospital. J Korean Med Sci. 2009. 24:585–91.
4). Mehrotra M., Wang G., Johnson WM. Multiplex PCR for detection of genes for Staphylococcus aureus enterotoxins, exfoliative toxins, toxic shock syndrome toxin 1, and methicillin resistance. J Clin Microbiol. 2000. 38:1032–5.
5). Johnson WM., Tyler SD., Ewan EP., Ashton FE., Pollard DR., Rozee KR. Detection of genes for enterotoxins, exfoliative toxins, and toxic shock syndrome toxin 1 in Staphylococcus aureus by the polymerase chain reaction. J Clin Microbiol. 1991. 29:426–30.
6). Ortega E., Abriouel H., Lucas R., Gáalvez A. Multiple Roles of Staphylococcus aureus enterotoxins: pathogenicity, super-antigenic activity, and correlation to antibiotic resistance. Toxins. 2010. 2:2117–31.
7). Becker K., Friedrich AW., Lubritz G., Weilert M., Peters G., Von Eiff C. Prevalence of genes encoding pyrogenic toxin super-antigens and exfoliative toxins among strains of Staphylococcus aureus isolated from blood and nasal specimens. J Clin Microbiol. 2003. 41:1434–9.
8). Diep BA., Carleton HA., Chang RF., Sensabaugh GF., Perdreau-Remington F. Roles of 34 virulence genes in the evolution of hospital- and community-associated strains of methicillin-resistant Staphylococcus aureus. J Infect Dis. 2006. 193:1495–503.
9). Hu DL., Omoe K., Inoue F., Kasai T., Yasujima M., Shinagawa K, et al. Comparative prevalence of superantigenic toxin genes in methicillin-resistant and methicillin-susceptible Staphylococcus aureus isolates. J Med Microbiol. 2008. 57:1106–12.
10). Novick RP. Mobile genetic elements and bacterial toxinoses: the superantigen-encoding pathogenicity islands of Staphylococcus aureus. Plasmid. 2003. 49:93–105.
11). Zaraket H., Otsuka T., Saito K., Dohmae S., Takano T., Higuchi W, et al. Molecular characterization of methicillin-resistant Staphylococcus aureus in hospitals in Niigata, Japan: divergence and transmission. Microbiol Immunol. 2007. 51:171–6.
12). Watanabe S., Ito I., Takeuchi E., Endo M., Okuno E., Hiramatsu K. Structural comparison of ten serotypes of staphylocoagulases in Staphylococcus aureus. J Bacteriol. 2005. 187:3698–707.
13). Kanemitsu K., Yamamoto H., Takemura H., Kaku M., Shimada J. Relatedness between the coagulase gene 3′-end region and coagulase serotypes among Staphylococcus aureus strains. Microbiol Immunol. 2001. 45:23–7.
14). Ishino K., Tsuchizaki N., Ishikawa J., Hotta K. Usefulness of PCR-Restriction Fragment Length Polymorphism typing of the coagulase gene to discriminate arbekacin-resistant Methicillin-Resistant Staphylococcus aureus strains. J Clin Microbiol. 2007. 45:607–9.
15). Hwang SM., Seki K., Sakurada J., Ogasawara M., Murai M., Ohmayu S, et al. Improved methods for detection and serotyping of coagulase from Staphylococcus aureus. Microbiol Immunol. 1989. 33:175–82.
16). Hisata K., Kuwahara-Arai K., Yamamoto M., Ito T., Nakatomi Y., Cui L, et al. Dissemination of methicillin-resistant staphylococci among healthy Japanese children. J Clin Microbiol. 2005. 43:3364–72.

17). Hanssen AM., Fossum A., Mikalsen J., Halvorsen DS., Bukholm G., Sollid JU. Dissemination of Community-acquired methicillin-resistant Staphylococcus aureus clones in Northern Norway: sequence types 8 and 80 predominate. J Clin Microbiol. 2005. 43:2118–24.
18). Collery MM., Smyth DS., Twohig JM., Shore AC., Coleman DC., Smyth CJ. Molecular typing of nasal carriage isolates of Staphylococcus aureus from an Irish university student population based on toxin gene PCR, agr locus types and multiple locus, variable number tandem repeat analysis. J Med Microbiol. 2008. 57:348–58.
19). Sila J., Sauer P., Kolar M. Comparison of the prevalence of genes coding for enterotoxins, exfoliatins, panton-valentine leukocidin and tsst-1 between methicillin-resistant and methicillin-susceptible isolates of Staphylococcus aureus at the university hospital in olomouc. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2009. 153:215–8.
20). Hwang SM., Kim TU. Changes in coagulase serotype of Staphylococcus aureus isolates in Busan, 1994-2005. Kor J Microbiol. 2007. 43:346–50.
21). Cha EK., Chang KS., Hwang SM. Correlation between staphylococcal cassette chromosome mec type and coagulase serotype of methicillin-resistant Staphylococcus aureus. J Bacteriol Viol. 2009. 39:71–8.
22). Kim JS., Song W., Kim HS., Cho HC., Lee KM., Choi MS, et al. Association between the methicillin resistance of clinical isolates of Staphylococcus aureus, their staphylococcal cassette chromosome mec (SCCmec) subtype classification, and their toxin gene profiles. Diagn Microbiol Infect Dis. 2006. 56:289–95.
23). Durand G., Bes M., Meugnier H., Enright MC., Forey F., Liassine N, et al. Detection of new methicillin-resistant Staphylococcus aureus clones containing the toxic shock syndrome toxin 1 gene responsible for hospital-and community-acquired infections in France. J Clin Microbiol. 2006. 44:847–53.
24). Kim JS., Kim HS., Song W., Cho HC., Lee KM., Kim EC. Molecular epidemiology of methicillin-resistant Staphylococcus aureus isolates with toxic shock syndrome toxin and staphy-lococcal enterotoxin C genes. Korean J Lab Med. 2007. 27:118–23.
25). Chini V., Dimitracopoulos G., Spiliopoulou I. Occurrence of the enterotoxin gene cluster and the toxic shock syndrome toxin 1 gene among clinical isolates of methicillin-resistant Staphylococcus aureus is related to clonal type and agr group. J Clin Microbiol. 2006. 44:1881–3.
26). Song JS., Choe PG., Song KH., Cho JH., Kim SH., Bang JH, et al. Multicenter study for frequency and clinical features of community-Associated methicillin-resistant Staphylococcus aureus in Korea. Infect Chemother. 2006. 38:325–33.
Go to : 
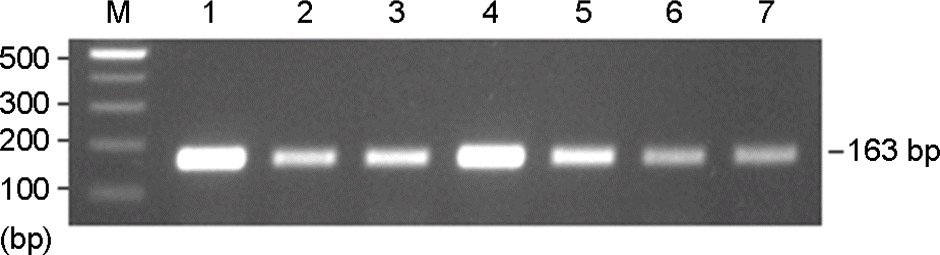 | Figure 1.Agarose gel analysis of PCR-amplified mecA genes from MRSA isolates. Lanes: M, 100 bp ladder; 1 to 7, MRSA isolates. |
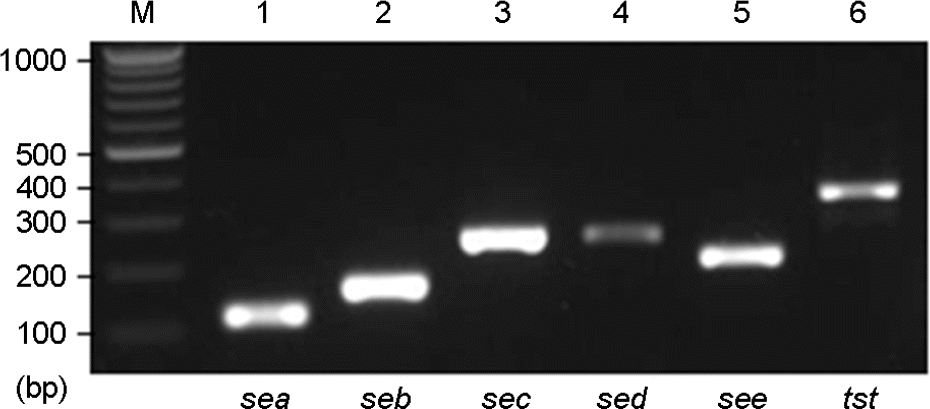 | Figure 2.Agarose gel analysis of PCR-amplified toxin genes from the standard strains of S. aureus. Lanes: M, 100 bp ladder; 1, sea (120 bp); 2, seb (164 bp); 3, sec (257 bp); 4, sed (278 bp); 5, see (209 bp); 6, tst (350 bp). |
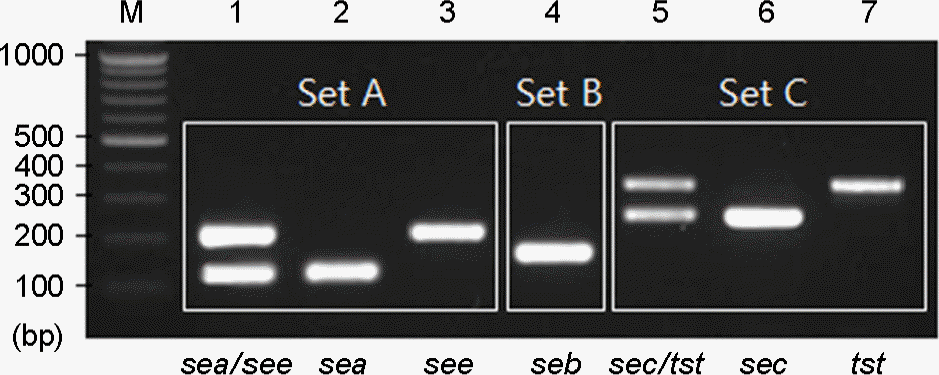 | Figure 3.Multiplex PCR patterns of toxin genes from S. aureus isolates. Lanes: M, 100 bp ladder; 1 to 3, primer set A; 4, primer set B; 5 to 7, primer set C. |
Table 1.
Primers for PCR amplification of toxin gene typing
Table 2.
Prevalence of SE and TSST-1 genes in S. aureus isolates
Table 3.
Combination of SE and TSST-1 genes in S. aureus isolates
Table 4.
Distribution of coagulase serotypes of S. aureus isolates
Table 5.
Correlation between prevalence of superantigenic toxin genes and coagulase serotypes in S. aureus isolates
| Toxin gene | No. of isolates | Coagulase serotype | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | VII | VIII | ND | ||
| sea | 4 | – | – | – | 3 (2)∗ | – | – | 1 | – | – |
| sea, seb | 1 | – | – | – | – | 1 | – | – | – | – |
| sea, seb, tst | 1 | – | – | – | – | – | – | 1 | – | – |
| sea, sec, tst | 1 | – | (1)∗ | – | – | – | – | – | – | – |
| sea, sec, see | 3 | – | – | – | – | – | – | 3 | – | – |
| sea, sec, see, tst | 1 | – | – | – | 1 | – | – | – | – | – |
| sea, see | 20 | – | – | – | 14 (4)∗ | – | – | 6 | – | – |
| sea, see, tst | 2 | – | – | – | 1 | – | – | 1 | – | – |
| sec | 5 | – | – | – | 1 | 1 | – | 1 | 1 | 1 |
| sec, tst | 13 | – | (13)∗ | – | – | – | – | – | – | – |
| tst | 8 | – | (5)∗ | – | – | 3 | – | – | – | – |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download