Abstract
Botulinum neurotoxin type A (BoNT/A) is a metalloprotease that cleaves SNAP-25 (synaptosome-associated protein of 25 kDa), a specific cellular protein essential for neurotransmitter release. As well as mouse bioassay to detect BoNT/A, various assay methods based on its endopeptidase activity have been developed. In this study, we tried to develop a BoNT/A assay system using recombinant SNAP-25 with glutathione S-transferase (GST) tags at both termini as substrate. The recombinant GST-SNAP-25-GST with 70 kDa was expressed and purified in E. coli and synthesized N-terminal 50 kDa and C-terminal 25 kDa fragment after cleavage at the Gln197-Arg198 bond by BoNT/A. To detect both fragments, we obtained rabbit antisera against peptides corresponding to the cleaved ends of each fragment. In the western blotting, the N-terminal fragment was detected by the antibody specifically recognizing the newly exposed C-terminus (corresponding to amino acid residue 191-197). This assay system was able to detect until 3.125 ng of BoNT/A, which corresponded to about 90 fold LD50 in mice. These results suggest that the in vitro endopeptidase assay developed in this study would replace others to detect BoNT/A.
Go to : 
REFERENCES
1). Centers for Disease Control and Prevention. Botulism in the United States: Handbook for epidemiologists, clinicians, and laboratory workers. Atlanta GA. Centers for Disease Control and Prevention. 1998.
2). Greenfield RA., Brown BR., Hutchins JB., Iandolo JJ., Jackson R., Slater LN., Bronze MS. Microbiological, biological, and chemical weapons of warfare and terrorism. Am J Med Sci. 2002. 323:326–40.

3). Franciosa G., Ferreira JL., Hatheway CL. Detection of type A, B, and E botulism neurotoxin genes in Clostridium botulinum and other Clostridium species by PCR: evidence of unexpressed type B toxin genes in type A toxigenic organisms. J Clin Microbiol. 1994. 32:1911–7.
4). Sugii S., Sakaguchi G. Botulogenic properties of vegetables with special reference to the molecular size of the toxin in them. J Food Safety. 1977. 1:53–65.

5). Sakaguchi G. Clostridium botulinum toxins. Pharmacol Ther. 1982. 19:165–94.
6). Binz T., Blasi J., Yamasaki S., Baumeister A., Link E., Sudhof TC., Jahn R., Niemann H. Proteolysis of SNAP-25 by type E and A botulinal neurotoxins. J Biol Chem. 1994. 269:1617–20.
7). Schiavo G., Matteoli M., Montecucco C. Neurotoxins affecting neuroexocytosis. Physiol Rev. 2000. 80:717–66.

8). Chung GT., Kang DH., Yoo CK., Choi JH., Seong WK. The first outbreak of botulism in Korea. Korean J Clin Microbiol. 2003. 6:160–3.
9). Yi HA., Lim JG., Lee JB., Her JH., Kim HA., Shin YE., Cho YW., Lee H., Yi SD. A familial outbreak of food-borne botulism. J Korean Neurol Assoc. 2004. 22:670–2.
10). Shin NR. Laboratory protocols for infection disease. Botulism. 3rd ed.Seoul: Korea National Institute of Health;2005. p. 369–77.
11). Shone C., Wilton-Smith P., Appleton N., Hambleton P., Modi N., Gatley S., Melling J. Monoclonal antibody-based immunoassay for type A Clostridium botulinum toxin is comparable to the mouse bioassay. Appl Environ Microbiol. 1985. 50:63–7.
12). Doellgast GJ., Triscott MX., Beard GA., Bottoms JD., Cheng T., Roh BH., Roman MG., Hall PA., Brown JE. Sensitive enzyme-linked immunosorbent assay for detection of Clostridium botulinum neurotoxins A, B, and E using signal amplification via enzyme-linked coagulation assay. J Clin Microbiol. 1993. 31:2402–9.
13). Sharma SK., Ferreira JL., Eblen BS., Whiting RC. Detection of type A, B, E, and F Clostridium botulinum neurotoxins in foods by using an amplified enzyme-linked immunosorbent assay with digoxigenin-labed antibodies. Appl Environ Microbiol. 2006. 72:1231–8.
14). Marion WR., O'Meara TE., Riddle GD., Berkhoff HA. Prevalence of Clostridium botulinum type C in substrates of phosphate-mine settling ponds and implication for epizootics of avian botulism. J Wildl Dis. 1983. 19:302–7.
15). Montecucco C., Schiavo G. Tetanus and botulism neurotoxins: a new group of zinc proteases. Trends Biochem Sci. 1993. 18:324–7.

16). Blasi J., Chapman ER., Link E., Binz T., Yamasaki S., De Camilli P., Sudhof TC., Niemann H., Jahn R. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature. 1993. 365:160–3.

17). Barr JR., Moura H., Boyer AE., Woolfitt AR., Kalb SR., Pavlopoulos A., McWilliams LG., Schmidt JG., Martinez RA., Ashley DL. Botulinum neurotoxin detection and differentiation by mass spectrometry. Emerg Infect Dis. 2005. 11:1578–83.

18). Ekong TA., Feavers IM., Sesardic D. Recombinant SNAP-25 is an effective substrate for Clostridium botulinum type A toxin endopeptidase activity in vitro. Microbiology. 1997. 143:3337–47.
19). Vaidyanathan VV., Yoshino K., Jahnz M., Dorries C., Bade S., Nauenburg S., Niemann H., Binz T. Proteolysis of SNAP-25 isoforms by botulinum neurotoxin type A, C, and E: Domains and amino acid residues controlling the formation of enzyme-substrate complexes and cleavage. J Neurochem. 1999. 72:327–37.
20). Hallis B., James BA., Shone CC. Development of novel assays for botulinum type A and B neurotoxins based on their endopeptidase activity. J Clin Microbiol. 1996. 34:1934–8.
21). Poras H., Ouimet T., Orng SV., Fournie-Zaluski MC., Popoff MR., Roques BP. Detection and quantification of botulinum neurotoxin type A by a novel rapid in vitro fluorimetric assay. Appl Environ Microbiol. 2009. 75:4382–90.
Go to : 
 | Figure 1.The schematic description of SNAP-25 sequence tagged with glutathione S-transferase (GST) at both termini. The number of amino acid residues corresponds to the sequence from native mouse SNAP-25. The peptide bond site of SNAP-25 cleaved by BoNT/A is between G197-A198 as indicated.in the SNAP-25. Two black boxes indicate the amino acid sequences of peptides synthesized to produce rabbit antisera. |
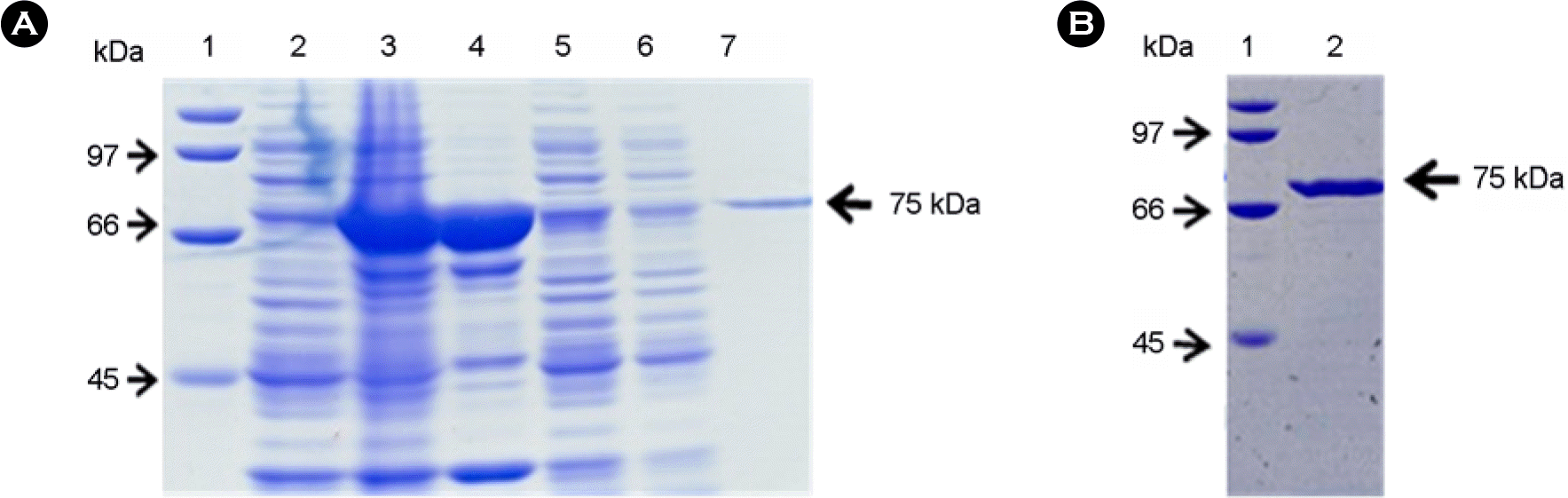 | Figure 2.The recombinant GST-SNAP-25-GST proteins in the expression and purification steps. (A) Samples from the each step were separated on 10% SDS-PAGE gel and visualized with Coomassie blue staining. Lane 1, protein markers; lane 2, transformed E. coli before IPTG induction; lane 3, transformed E. coli after IPTG induction for 4 h; lane 4, a sonicated bacterial pellet; lane 5, input sample for glutathione affinity; lane 6, flow-through in glutathione affinity; lane 7, GST-SNAP-25-GST purified with glutathione bead. (B) The purity of recombinant GST-SNAP-25-GST was confirmed by SDS-PAGE. Recombinant GST-SNAP-25-GST purified with glutathione bead was buffer-changed by dialysis and concentrated. Lane 1, protein markers; lane 2, the final product of recombinant GST-SNAP-25-GST purified from culture supernatant of transformed E. coli. |
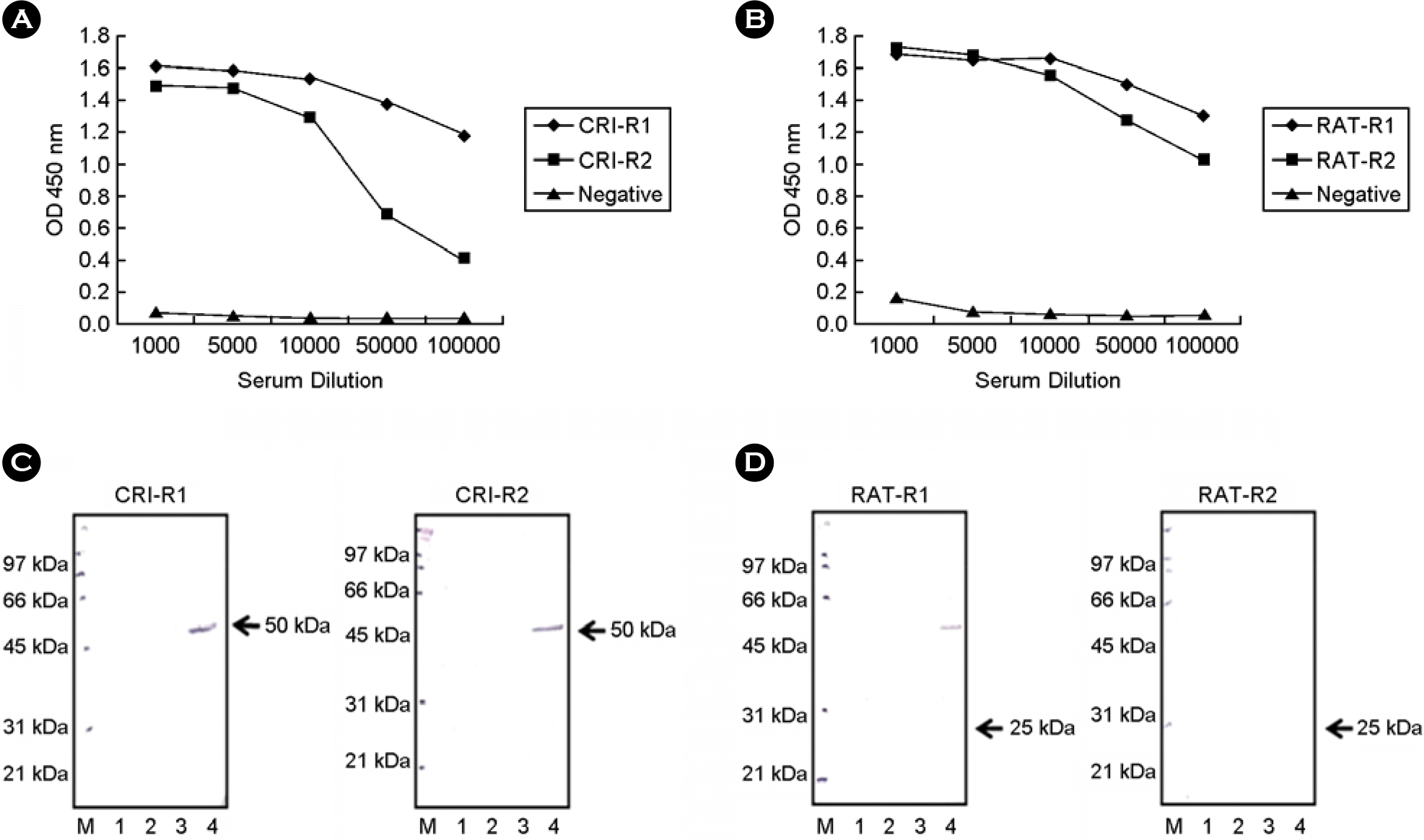 | Figure 3.Efficacy measurement of anti-SNAP-25 antisera. The antisera were raised against peptides around newly exposed termini of the SNAP-25 fragments cleaved by BoNT/A from rabbits. (A) ELISA analysis of CRI-R1 and CRI-R2 antisera. These antisera were raised against peptides around newly exposed terminus of N-terminal SNAP-25 fragment by toxin. (B) ELISA analysis of RAT-R1 and RAT-R2 antisera. These antisera were raised against peptides around newly exposed terminus of C-terminal SNAP-25 fragment by toxin. Western blot analysis with CRI-R1 and CRI-R2 (C) and RAT-R1 and RAT-R2 (D) antisera after endopeptidase assay. Forty micromoles of GST-SNAP-25-GST were reacted with 360 μg of BoNT/A in 10 μl for 4 h at 37°C, separated in 10% SDS-PAGE, and analyzed by western blotting with sera. The antisera detected about 50 kDa N-terminal fragment of SNAP-25 cleaved by active BoNT/A. GST (3 μg) was used as a negative control. Lane M, protein markers; lane 1, GST only; lane 2, GST reacted with BoNT/A; lane 3, GST-SNAP-25-GST only; Lane 4, GST-SNAP-25-GST reacted with BoNT/A. |
 | Figure 4.Detective sensitivity of BoNT/A in the endopeptidase assay using GST-SNAP-25-GST. (A) SDS-PAGE analysis. Forty micromoles of GST-SNAP-25-GST were reacted with various concentrations of BoNT/A in 10 μl for 4 h at 37°C, separated in 10% SDS-PAGE and visualized with Coomassie blue staining to detect 50 kDa N-terminal fragment of SNAP-25 (arrow). (B) Western blot analysis with anti-SNAP-25 antiserum. After the reaction by BoNT/A with the indicated concentrations and separation in SDS-PAGE with the same procedure described in (A), the products of GST-SNAP-25-GST were detected with CRI-R1 antiserum, which detect only 50 kDa N-terminal fragment of SNAP-25 (arrow). The undigested full-length GST-SNAP-25-GST was detected weakly around 75 kDa. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download