Abstract
Bifidobacteria is one of the prototypes of probiotics bacteria, normally inhabitating the intestinal tract of humans. To search for a potent immunoregulatory Bifidobacteria strain, we screened the Bifidobacteria strains isolated from the feces of healthy Korean children. The mRNA or protein expression of an anti-inflammatory cytokine, IL-10, from mouse macrophages stimulated with live Bifidobacteria was examined. Of tested strains, Bifidobacteria A28 induced the highest IL-10 gene expression of murine macrophages. To probe immunoregulatory activity of the selected strain on the mice, we evaluated the proportional changes of CD4+CD25+ surface marker in the murine splenocytes. Flow cytometric analysis showed that the overall percentages of CD4+CD25+ cells in A28-treated splenocytes were higher than those of untreated splenocytes. In parallel, IL-10 release from A28-treated mouse peritoneal macrophages and splenocytes was significantly higher than that of untreated control cells. Collectively, the Bifidobacteria A28 strain isolated from the feces of healthy Korean children augments the mRNA or protein expression of IL-10 release from mouse peritoneal macrophages as well as the proportion of CD4+CD25+ cells of naïve splenocytes. These provide in vitro scientific clues that Bifidobacteria A28 might be usable for anti-inflammatory disease such as inflammatory bowel disease (IBD).
Go to : 
REFERENCES
1). Poupard JA., Husain I., Norris RF. Biology of the bifidobacteria. Bacteriol Rev. 1973. 37:136–65.

2). Rhee YK., Han MJ., Choi EC., Kim DH. Hypocholesterolemic activity of Bifidobacteria isolated from a healthy Korean. Arch Pharm Res. 2002. 25:681–4.
3). Sekine K., Ohta J., Onishi M., Tatsuki T., Shimokawa Y., Toida T, et al. Analysis of antitumor properties of effector cells stimulated with a cell wall preparation (WPG) of Bifidobacterium infantis. Biol Pharm Bull. 1995. 18:148–53.
4). Hatcher GE., Lambrecht RS. Augmentation of macrophage phagocytic activity by cell-free extracts of selected lactic acid-producing bacteria. J Dairy Sci. 1993. 76:2485–92.

5). Sekine K., Watanabe-Sekine E., Toida T., Kasashima T., Kataoka T., Hashimoto Y. Adjuvant activity of the cell wall of Bifidobacterium infantis for in vivo immune responses in mice. Immunopharmacol Immunotoxicol. 1994. 16:589–609.
6). Hart AL., Lammers K., Brigidi P., Vitali B., Rizzello F., Gionchetti P, et al. Modulation of human dendritic cell phenotype and function by probiotic bacteria. Gut. 2004. 53:1602–9.

7). Park JH., Um JI., Lee BJ., Goh JS., Park SY., Kim WS, et al. Encapsulated Bifidobacterium bifidum potentiates intestinal IgA production. Cell Immunol. 2002. 219:22–7.
8). Bernet MF., Brassart D., Neeser JR., Servin AL. Adhesion of human bifidobacterial strains to cultured human intestinal epithelial cells and inhibition of enteropathogen-cell interactions. Appl Environ Microbiol. 1993. 59:4121–8.

9). Fedorak RN., Madsen KL. Probiotics and the management of inflammatory bowel disease. Inflamm Bowel Dis. 2004. 10:286–99.

10). Salminen S., Salminen E. Lactulose lactic acid bacteria, intestinal microecology and mucosal protection. Scand J Gastroenterol Suppl. 1997. 222:45–8.

11). Boulinier G. Arthur Keith and the first settlement of human being in Malta. Two subversive teeth. Hist Sci Med. 2004. 38:37–48.
12). He F., Morita H., Ouwehand AC., Hosoda M., Hiramatsu M., Kurisaki J, et al. Stimulation of the secretion of pro-inflammatory cytokines by Bifidobacterium strains. Microbiol Immunol. 2002. 46:781–5.
13). Park SY., Ji GE., Ko YT., Jung HK., Ustunol Z., Pestka JJ. Potentiation of hydrogen peroxide, nitric oxide, and cytokine production in RAW 264.7 macrophage cells exposed to human and commercial isolates of Bifidobacterium. Int J Food Microbiol. 1999. 46:231–41.
14). Young SL., Simon MA., Baird MA., Tannock GW., Bibiloni R., Spencely K, et al. Bifidobacterial species differentially affect expression of cell surface markers and cytokines of dendritic cells harvested from cord blood. Clin Diagn Lab Immunol. 2004. 11:686–90.

15). Moore KW., de Waal Malefyt R., Coffman RL., O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001. 19:683–765.

16). Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004. 22:531–62.
17). Shevach EM. CD4+CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002. 2:389–400.
18). Niers LE., Timmerman HM., Rijkers GT., van Bleek GM., van Uden NO., Knol EF, et al. Identification of strong interleukin-10 inducing lactic acid bacteria which down-regulate T helper type 2 cytokines. Clin Exp Allergy. 2005. 35:1481–9.

19). Gill HS., Rutherfurd KJ., Cross ML., Gopal PK. Enhancement of immunity in the elderly by dietary supplementation with the probiotic Bifidobacterium lactis HN019. Am J Clin Nutr. 2001. 74:833–9.
Go to : 
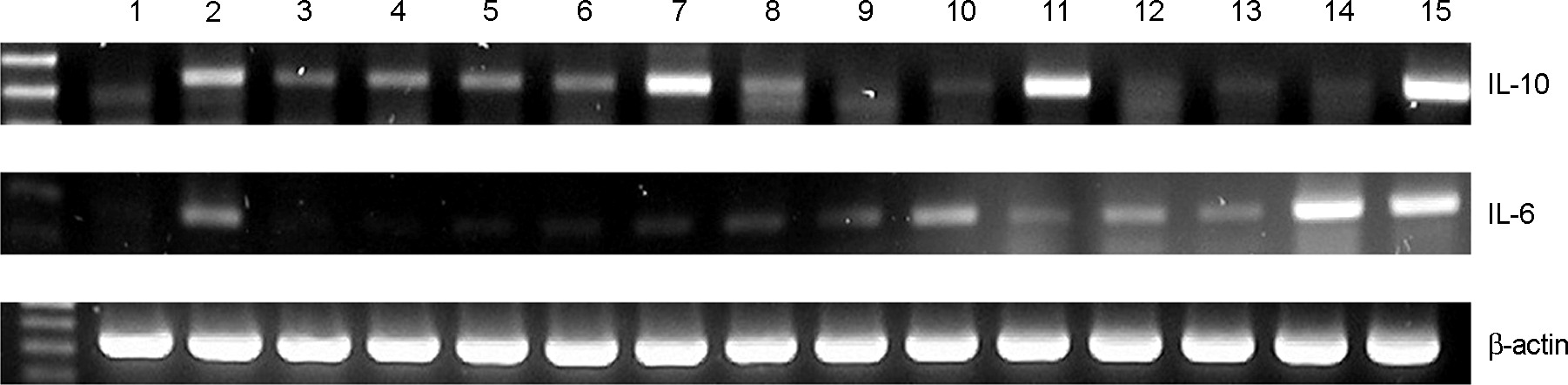 | Figure 1.
Expression of IL-10 mRNA in RAW 264.7 cells treated with various Bifidus strains. Lane 1, control; Lane 2, LPS 100 ng/ml; Lane 3, B. infantis; Lane 4, B. catenulactum; Lane 5, A1; Lane 6, A2; Lane 7, A5; Lane 8, A13; Lane 9, A14; Lane 10, A16; Lane 11, A28; Lane 12, A34; Lane 13, B17; Lane 14, B2730; Lane 15, YM112 |
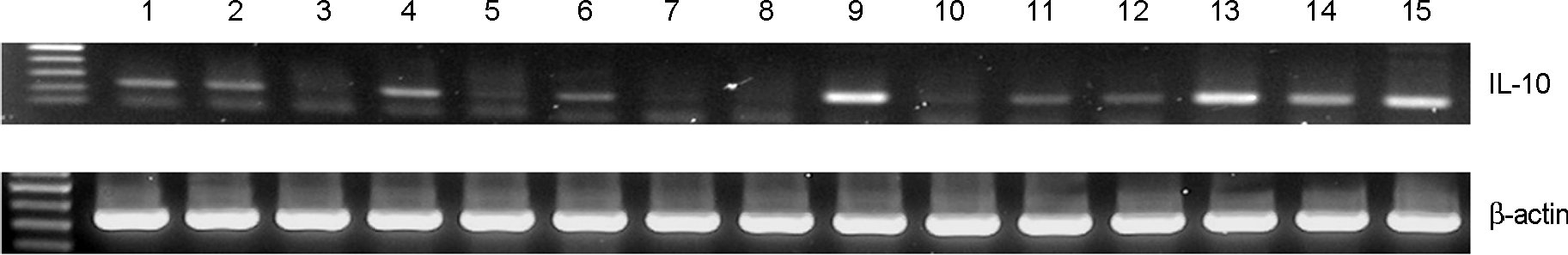 | Figure 2.
Expression of IL-10 mRNA in mouse peritoneal macrophage treated with various Bifidus strains. Lane 1, control; Lane 2, LPS 100 ng/ml; Lane 3, A1; Lane 4, A2; Lane 5, A5; Lane 6, A13; Lane 7, A14; Lane 8, A16; Lane 9, A28; Lane 10, A34; Lane 11, B17; Lane 12, B2730; Lane 13, YM112; Lane 14, B. infantis; Lane 15, B. catenulactum
|
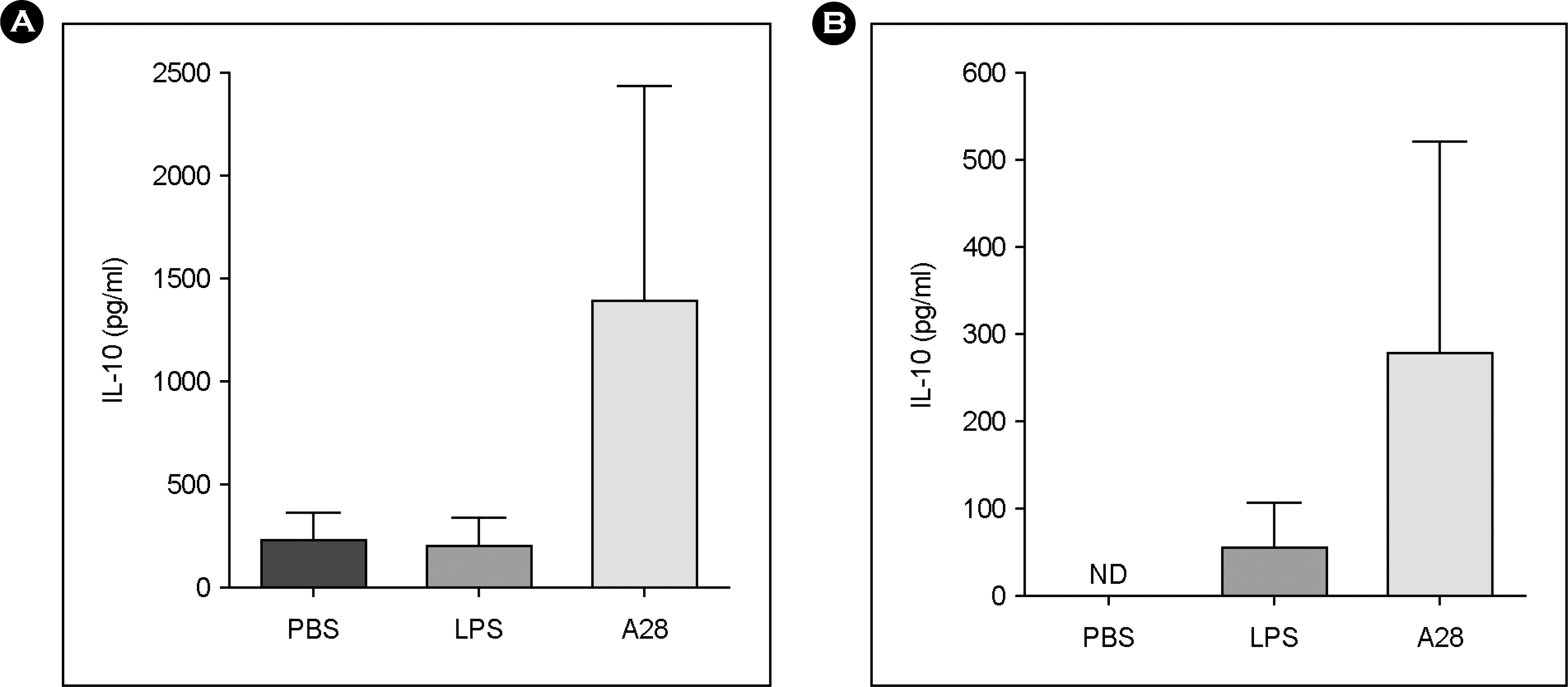 | Figure 3.Production of IL-10 by mouse peritoneal macrophages (A) and splenocytes (B) treated with Bifidus strain A28. |
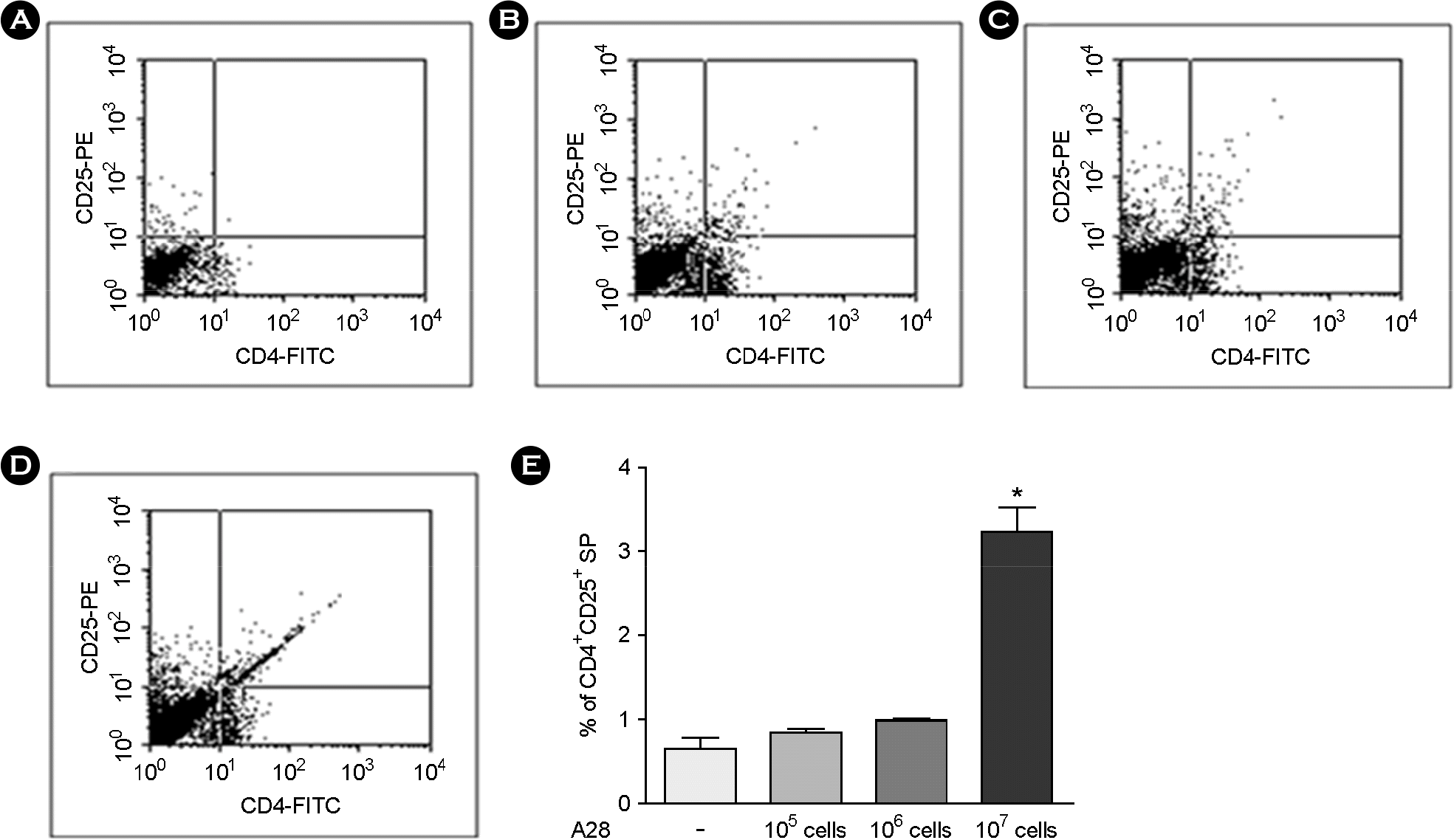 | Figure 4.
Proportional increment of CD4+CD25+ T cell in mouse splenocytes treated with Bifidus strain A28. A: CD4+CD25+ expression in A28-untretaed mouse splenocytes; B: CD4+CD25+ expression in A28 (105 cells/ml)-treated mouse splenocytes; C: CD4+CD25+ expression in A28 (106 cells/ml)-treated mouse splenocytes; D: CD4+CD25+ expression in A28 (107 cells/ml)-treated mouse splenocytes; E: Comparative CD4+CD25+ expression in different numbers of A28-treated mouse splenocytes. Data were expressed as Mean ± standard error and analyzed with one-way ANOVA (∗p < 0.05). |
Table 1.
Primer sequences and sizes for PCR




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download