Abstract
Orientia tsutsugamushi, a causative agent of scrub typhus, is an obligate intracellular parasite and usually propagates in the cytoplasm of host endothelial cells and macrophages. Macrophages are the first defense line against bacterial infection and NF-κB is activated upon contact with bacteria, resulting in the transcription of inflammatory cytokine to control bacterial infection. In this study, we investigated whether O. tsutsugamushi modulates NF-κB activation in the macrophages. We examined the changes of NF-κB proteins upon infection with O. tsutsugamushi and found that NF-κB is activated at a slow rate as judged with EMSA and immunoblot analysis. Interestingly, we found that p65 was cleaved generating a 45 kDa fragment. In addition, fragment of p65 is generated only by the virulent serotype strain of O. tsutsugamushi, suggesting this cleavage may be associated with the mouse virulence. It is still unknown whether this is a direct result of O. tsutsugamushi proteins or enzymes of host cell. Further exploration of the mechanism that modulates NF-κB activity by O. tsutsugamushi could contribute to a better understanding of the molecular pathogenesis of O. tsutsugamushi infection.
Go to : 
REFERENCES
1). Seong SY., Choi MS., Kim IS. Orientia tsutsugamushi infection: overview and immune responses. Microbes Infect. 2001. 3:11–21.
2). Cho KA., Jun YH., Suh JW., Kang JS., Choi HJ., Woo SY. Orientia tsutsugamushi induced endothelial cell activation via the NOD1-IL-32 pathway. Microb Pathog. 2010. 49:95–104.
3). Moron CG., Popov VL., Feng HM., Wear D., Walker DH. Identification of the target cells of Orientia tsutsugamushi in human cases of scrub typhus. Mod Pathol. 2001. 14:752–9.
4). Kelly DJ., Fuerst PA., Ching WM., Richards AL. Scrub typhus: the geographic distribution of phenotypic and genotypic variants of Orientia tsutsugamushi. Clin Infect Dis. 2009. 48:S203–30.
5). Kweon SS., Choi JS., Lim HS., Kim JR., Kim KY., Ryu SY, et al. Rapid increase of scrub typhus, South Korea, 2001-2006. Emerg Infect Dis. 2009. 15:1127–9.

6). Hwang TS., Chu YC., Kim YB., Lim BU., Kang JS. Pathologic study of mice infected with Rickettsia tsutsugamushi R19 strain. J Korean Med Sci. 1993. 8:437–45.
7). Kumar H., Kawai T., Akira S. Toll-like receptors and innate immunity. Biochem Biophys Res Commun. 2009. 388:621–5.

8). Naumann M. Nuclear Factor-kappa B activation and innate immune response in microbial pathogen infection. Biochem Pharmacol. 2000. 60:1109–14.
9). Li Q., Verma IM. NF-kappa B regulation in the immune system. Nat Rev Immunol. 2002. 2:725–34.
10). al-Ramadi BK., Chen YW., Meissler JJ Jr., Eisenstein TK. Immunosuppression induced by attenuated Salmonella. Reversal by IL-4. J Immunol. 1991. 147:1954–61.
11). Kim MJ., Kim MK., Kang JS. Orientia tsutsugamushi inhibits tumor necrosis factor alpha production by inducing interleukin 10 secretion in murine macrophages. Microb Pathog. 2006. 40:1–7.
12). Cho NH., Seong SY., Huh MS., Han TH., Koh YS., Choi MS, et al. Expression of chemokine genes in murine macrophages infected with Orientia tsutsugamushi. Infect Immun. 2000. 68:594–602.
13). Zhong H., May MJ., Jimi E., Ghosh S. The phosphorylation status of nuclear NF-kappa B determines its association with CBP/p300 or HDAC-1. Mol Cell. 2002. 9:625–36.
14). Kim MK., Kang JS. Orientia tsutsugamushi suppresses the production of inflammatory cytokines induced by its own heat-stable component in murine macrophages. Microb Pathog. 2001. 31:145–50.
15). Tato CM., Hunter CA. Host-pathogen interactions: subversion and utilization of the NF-kappa B pathway during infection. Infect Immun. 2002. 70:3311–7.
16). Silverman N., Maniatis T. NF-kappa B signaling pathways in mammalian and insect innate immunity. Genes Dev. 2001. 15:2321–42.
17). Sporn LA., Sahni SK., Lerner NB., Marder VJ., Silverman DJ., Turpin LC, et al. Rickettsia rickettsii infection of cultured human endothelial cells induces NF-kappaB activation. Infect Immun. 1997. 65:2786–91.
18). Lin M., Rikihisa Y. Ehrlichia chaffeensis downregulates surface Toll-like receptors 2/4, CD14 and transcription factors PU.1 and inhibits lipopolysaccharide activation of NF-kappa B, ERK 1/2 and p38 MAPK in host monocytes. Cell Microbiol. 2004. 6:175–86.
19). Jerrells TR., Geng P. The role of tumor necrosis factor in host defense against scrub typhus rickettsiae. II. Differential induction of tumor necrosis factor-alpha production by Rickettsia tsutsugamushi and Rickettsia conorii. Microbiol Immunol. 1994. 38:713–9.
20). Kim HS., Chang I., Kim JY., Choi KH., Lee MS. Caspase-mediated p65 cleavage promotes TRAIL-induced apoptosis. Cancer Res. 2005. 65:6111–9.

21). Neuzil J., Schröder A., von Hundelshausen P., Zernecke A., Weber T., Gellert N, et al. Inhibition of inflammatory endothelial responses by a pathway involving caspase activation and p65 cleavage. Biochemistry. 2001. 40:4686–92.

22). Neznanov N., Chumakov KM., Neznanova L., Almasan A., Banerjee AK., Gudkov AV. Proteolytic cleavage of the p65-RelA subunit of NF-kappa B during poliovirus infection. J Biol Chem. 2005. 280:24153–8.
23). Lad SP., Li J., da Silva Correia J., Pan Q., Gadwal S., Ulevitch RJ, et al. Cleavage of p65/RelA of the NF-kappaB pathway by Chlamydia. Proc Natl Acad Sci USA. 2007. 104:2933–8.
24). Cho NH., Kim HR., Lee JH., Kim SY., Kim J., Cha S, et al. The Orientia tsutsugamushi genome reveals massive proliferation of conjugative type IV secretion system and host-cell interaction genes. Proc Natl Acad Sci USA. 2007. 104:7981–6.
Go to : 
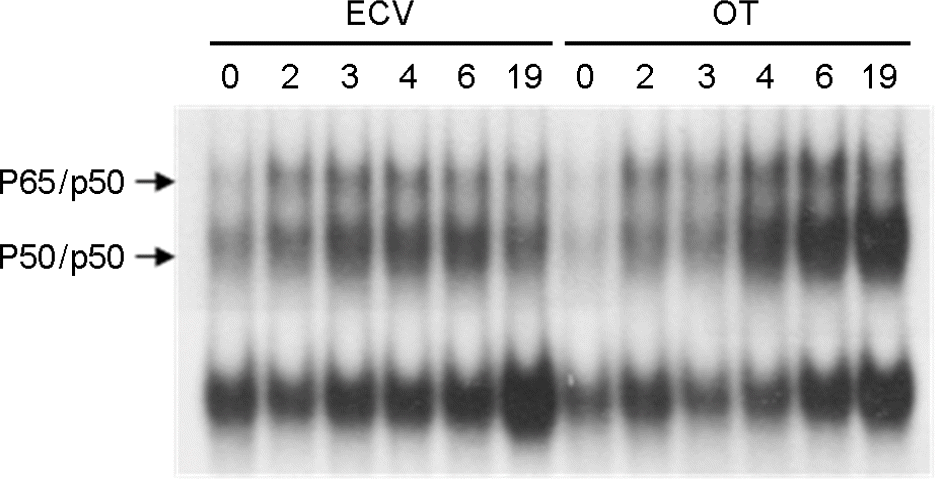 | Figure 1.The activation of NF-κB in J774A.1 cells infected with O. tsutsugamushi. NF-κB activation was examined by EMSA for nuclear extracts prepared from J774A.1 cells for the indicated times. J774A.1 cells were infected with O. tsutsugamushi (OT) or treated ECV cell lysate for the mock-infection (ECV). |
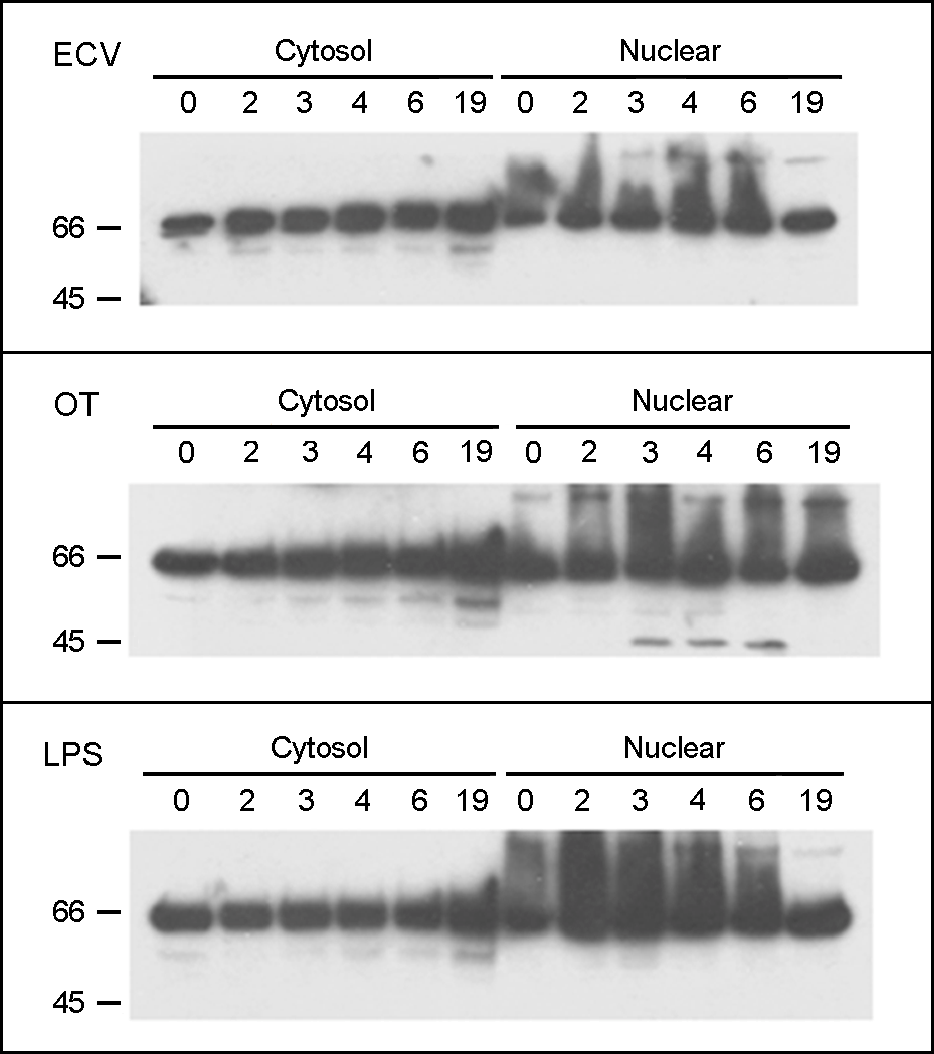 | Figure 2.The cleavage of p65 in J774A.1 cells infected with O. tsutsugamushi. The p65 was visualized with the immunoblot analysis of protein extracts from the cytosol and nuclei of the J774A.1 cells treated ECV cell lysate (upper panel), infected with O. tsutsugamushi (middle panel), or treated with LPS (lower panel) for the indicated times. |
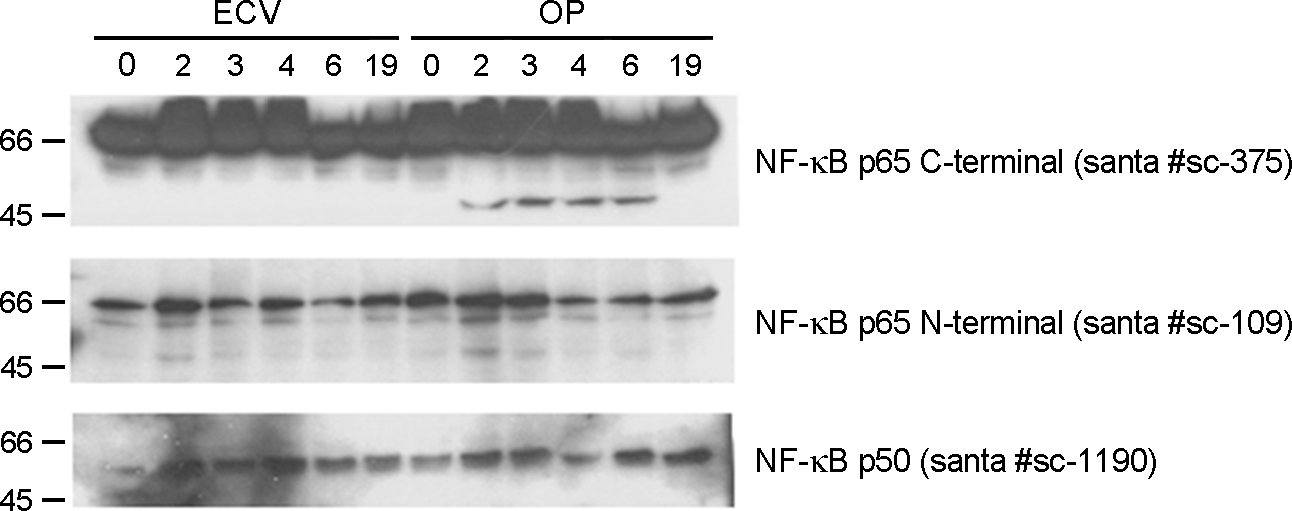 | Figure 3.The cleavage of p65 observed only in western blot using p65 C- and N-terminal antibodies. The p65 was visualized with the immunoblot analysis of protein extracts from the nuclei of J774A.1 cells treated ECV cell lysate (ECV) and infected with O. tsutsugamushi (OT) using the indicated primary antibodies. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download